Monosaccharides, also called simple sugars, are the simplest form of sugar and the most basic units of carbohydrates. They cannot be further hydrolyzed to simpler chemical compounds. The general formula is C
nH
2nO
n. They are usually colorless, water-soluble, and crystalline solids. Some monosaccharides have a sweet taste.

Optical rotation or optical activity is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Optical activity occurs only in chiral materials, those lacking microscopic mirror symmetry. Unlike other sources of birefringence which alter a beam's state of polarization, optical activity can be observed in fluids. This can include gases or solutions of chiral molecules such as sugars, molecules with helical secondary structure such as some proteins, and also chiral liquid crystals. It can also be observed in chiral solids such as certain crystals with a rotation between adjacent crystal planes or metamaterials. Rotation of light's plane of polarization may also occur through the Faraday effect which involves a static magnetic field, however this is a distinct phenomenon that is not usually classified under "optical activity."
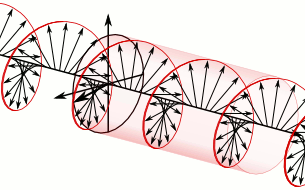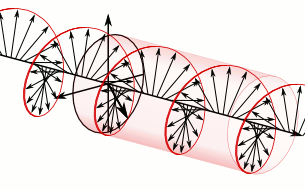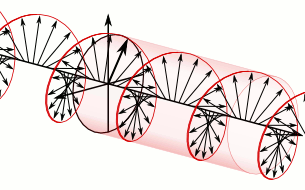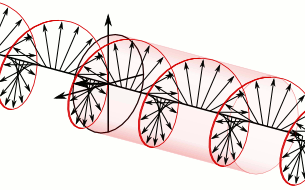
In electrodynamics, circular polarization of an electromagnetic wave is a polarization state in which, at each point, the electric field of the wave has a constant magnitude but its direction rotates with time at a steady rate in a plane perpendicular to the direction of the wave.

A mirror image is a reflected duplication of an object that appears almost identical, but is reversed in the direction perpendicular to the mirror surface. As an optical effect it results from reflection off of substances such as a mirror or water. It is also a concept in geometry and can be used as a conceptualization process for 3-D structures.

In chemistry, an enantiomer, also known as an optical isomer, is one of two stereoisomers that are mirror images of each other that are non-superposable, much as one's left and right hands are the same except for being reversed along one axis. A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph ; the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may be some perfect-mirror-image pairs.
In a molecule, a stereocenter is a particular instance of a stereogenic element that is geometrically a point. A stereocenter or stereogenic center is any point in a molecule, though not necessarily an atom, bearing groups, such that an interchanging of any two groups leads to a stereoisomer. The term stereocenter was introduced in 1984 by Kurt Mislow and Jay Siegel. A chiral center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superimposable on its mirror image. The concept of a chiral center generalizes the concept of an asymmetric carbon atom such that an interchanging of any two groups gives rise to an enantiomer. In organic chemistry, a chiral center usually refers to a carbon, phosphorus, or sulfur atom, though it is also possible for other atoms to be chiral centers, especially in areas of organometallic and inorganic chemistry.
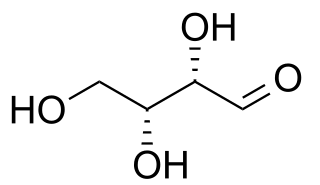
Diastereomers are a type of a stereoisomer. Diasteoreomers are defined as non-mirror image non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other.
When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.

A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereogenic centers, the molecule is not chiral. A meso compound is "superposable" on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter.

A metamaterial is a material engineered to have a property that is not found in naturally occurring materials. They are made from assemblies of multiple elements fashioned from composite materials such as metals or plastics. The materials are usually arranged in repeating patterns, at scales that are smaller than the wavelengths of the phenomena they influence. Metamaterials derive their properties not from the properties of the base materials, but from their newly designed structures. Their precise shape, geometry, size, orientation and arrangement gives them their smart properties capable of manipulating electromagnetic waves: by blocking, absorbing, enhancing, or bending waves, to achieve benefits that go beyond what is possible with conventional materials.
Handedness is a human attribute reflecting the unequal distribution of fine motor skill between the left and right hands.
Optical rotatory dispersion is the variation in the optical rotation of a substance with a change in the wavelength of light. Optical rotatory dispersion can be used to find the absolute configuration of metal complexes. For example, when plane-polarized white light from an overhead projector is passed through a cylinder of sucrose solution, a spiral rainbow is observed perpendicular to the cylinder.
In the mathematical field of knot theory, a chiral knot is a knot that is not equivalent to its mirror image. An oriented knot that is equivalent to its mirror image is an amphichiral knot, also called an achiral knot or amphicheiral knot. The chirality of a knot is a knot invariant. A knot's chirality can be further classified depending on whether or not it is invertible.
In chemistry, isomers are ions or molecules with identical formulas but distinct structures. Isomers do not necessarily share similar properties. Two main forms of isomerism are structural isomerism and stereoisomerism.
In chemistry, the term supramolecular chirality is used to describe supramolecular assemblies that are non-superposable on their mirror images.
Metachirality is a stronger form of chirality.
It applies to objects or systems that are chiral and where, in addition, their mirror image has a symmetry group that differs from the symmetry group of the original object or system.
Mirror life is a hypothetical form of life with mirror-reflected molecular building blocks. The possibility of mirror life was first discussed by Louis Pasteur. Although this alternative life form has not been discovered in nature, efforts to build a mirror-image version of biology's molecular machinery are already underway.









