Related Research Articles
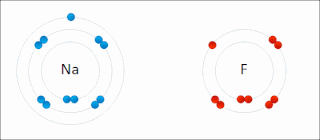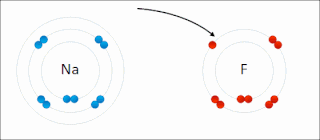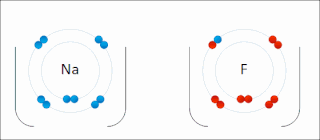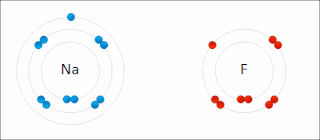
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding, along with covalent bonding and metallic bonding. Ions are atoms with an electrostatic charge. Atoms that gain electrons make negatively charged ions. Atoms that lose electrons make positively charged ions. This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like NH+
4 or SO2−
4. In simpler words, an ionic bond results from the transfer of electrons from a metal to a non-metal in order to obtain a full valence shell for both atoms.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

In chemistry, a reagent or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms reactant and reagent are often used interchangeably, but reactant specifies a substance consumed in the course of a chemical reaction. Solvents, though involved in the reaction mechanism, are usually not called reactants. Similarly, catalysts are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
A substitution reaction is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
![<span class="mw-page-title-main">Chemical ionization</span> Ionization technique used in mass [[spectroscopy]]](https://upload.wikimedia.org/wikipedia/commons/thumb/7/7b/Chemical_Ionization.png/320px-Chemical_Ionization.png)
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules are ionized by electron ionization to form reagent ions, which subsequently react with analyte molecules in the gas phase to create analyte ions for analysis by mass spectrometry. Negative chemical ionization (NCI), charge-exchange chemical ionization, atmospheric-pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) are some of the common variants of the technique. CI mass spectrometry finds general application in the identification, structure elucidation and quantitation of organic compounds as well as some utility in biochemical analysis. Samples to be analyzed must be in vapour form, or else, must be vapourized before introduction into the source.

A chemical substance is a form of matter having constant chemical composition and characteristic properties. Chemical substances can be simple substances, chemical compounds, or alloys.
Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule.
Fluorogenic describes a property of chemical compounds which are initially not fluorescent, but become fluorescent through a chemical reaction, typically through an intermolecular covalent reaction which binds the now fluorescent compound to a target molecule. IUPAC uses a broader definition of fluorogenic, wherein a enhancement of fluorescence via a chemical reaction is not required, however in contrast to the IUPAC definition common use of fluorogenic does not refer to non-reaction effects like the enhancement of fluorescence from a fluorophore being in different solvents. Fluorogenic labeling reagents are often used in analytical chemistry procedures, particularly in HPLC or CE to derivative target compounds, thereby allowing enhanced sensitivity through fluorescence based detection.

Spin trapping is an analytical technique employed in chemistry and biology for the detection and identification of short-lived free radicals through the use of electron paramagnetic resonance (EPR) spectroscopy. EPR spectroscopy detects paramagnetic species such as the unpaired electrons of free radicals. However, when the half-life of radicals is too short to detect with EPR, compounds known as spin traps are used to react covalently with the radical products and form more stable adduct that will also have paramagnetic resonance spectra detectable by EPR spectroscopy. The use of radical-addition reactions to detect short-lived radicals was developed by several independent groups by 1968.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.
In chemistry, a color reaction or colour reaction is a chemical reaction that is used to transform colorless chemical compounds into colored derivatives which can be detected visually or with the aid of a colorimeter.
DNA-encoded chemical libraries (DECL) is a technology for the synthesis and screening on an unprecedented scale of collections of small molecule compounds. DECL is used in medicinal chemistry to bridge the fields of combinatorial chemistry and molecular biology. The aim of DECL technology is to accelerate the drug discovery process and in particular early phase discovery activities such as target validation and hit identification.
In organic chemistry, diazirines are a class of organic molecules consisting of a carbon bound to two nitrogen atoms, which are double-bonded to each other, forming a cyclopropene-like ring, 3H-diazirene. They are isomeric with diazocarbon groups, and like them can serve as precursors for carbenes by loss of a molecule of dinitrogen. For example, irradiation of diazirines with ultraviolet light leads to carbene insertion into various C−H, N−H, and O−H bonds. Hence, diazirines have grown in popularity as small, photo-reactive, crosslinking reagents. They are often used in photoaffinity labeling studies to observe a variety of interactions, including ligand-receptor, ligand-enzyme, protein-protein, and protein-nucleic acid interactions.
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes, between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.
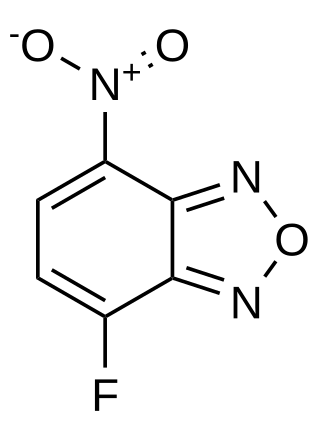
4-Fluoro-7-nitrobenzofurazan (NBD-F) is a fluorogenic, amine labeling dye that is not fluorescent itself, but covalently reacts with secondary or primary amines to form a fluorescently labeled product. It and other fluorogenic benzofurans are used for derivitization in HPLC applications. After the fluorogenic reaction, it can be detected with an excitation wavelength of 470 nm (blue) and an emission wavelength of 530 nm (green), enabling an HPLC limit of detection of 10 fmol.

3-(4-carboxybenzoyl)quinoline-2-carboxaldehyde (CBQCA) is a fluorogenic amine labeling dye that is not fluorescent itself, but covalently reacts with primary amines to form fluorescent products. It was first reported in 1991. Today, it is largely used in the context of quantifying peptides or proteins. Either cyanide or thiols are required as a co-substrate in the fluorogenic reaction, although thiols also react with & mask the CBQCA aldehyde thereby preventing the fluorogenic reaction against the targeted primary amines. Once bound to protein the excitation wavelength is 465 nm (blue) and the emission wavelength is ~550 nm (green).
References
- ↑ Fanali, Salvatore; Haddad, Paul R.; Poole, Colin; Riekkola, Marja-Liisa (2017). Liquid chromatography. Volume 2, Applications. Amsterdam, Netherlands. ISBN 978-0-12-809344-3. OCLC 992119225.