Related Research Articles

The compression ratio is the ratio between the volume of the cylinder and combustion chamber in an internal combustion engine at their maximum and minimum values.

An engine or motor is a machine designed to convert one or more forms of energy into mechanical energy.

A heat engine is a system that converts heat to usable energy, particularly mechanical energy, which can then be used to do mechanical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, particularly electrical, since at least the late 19th century. The heat engine does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag.

A reciprocating engine, also often known as a piston engine, is typically a heat engine that uses one or more reciprocating pistons to convert high temperature and high pressure into a rotating motion. This article describes the common features of all types. The main types are: the internal combustion engine, used extensively in motor vehicles; the steam engine, the mainstay of the Industrial Revolution; and the Stirling engine for niche applications. Internal combustion engines are further classified in two ways: either a spark-ignition (SI) engine, where the spark plug initiates the combustion; or a compression-ignition (CI) engine, where the air within the cylinder is compressed, thus heating it, so that the heated air ignites fuel that is injected then or earlier.

In internal combustion engines, exhaust gas recirculation (EGR) is a nitrogen oxide (NOx) emissions reduction technique used in petrol/gasoline, diesel engines and some hydrogen engines. EGR works by recirculating a portion of an engine's exhaust gas back to the engine cylinders. The exhaust gas displaces atmospheric air and reduces O2 in the combustion chamber. Reducing the amount of oxygen reduces the amount of fuel that can burn in the cylinder thereby reducing peak in-cylinder temperatures. The actual amount of recirculated exhaust gas varies with the engine operating parameters.

A four-strokeengine is an internal combustion (IC) engine in which the piston completes four separate strokes while turning the crankshaft. A stroke refers to the full travel of the piston along the cylinder, in either direction. The four separate strokes are termed:
- Intake: Also known as induction or suction. This stroke of the piston begins at top dead center (T.D.C.) and ends at bottom dead center (B.D.C.). In this stroke the intake valve must be in the open position while the piston pulls an air-fuel mixture into the cylinder by producing a partial vacuum in the cylinder through its downward motion.
- Compression: This stroke begins at B.D.C, or just at the end of the suction stroke, and ends at T.D.C. In this stroke the piston compresses the air-fuel mixture in preparation for ignition during the power stroke (below). Both the intake and exhaust valves are closed during this stage.
- Combustion: Also known as power or ignition. This is the start of the second revolution of the four stroke cycle. At this point the crankshaft has completed a full 360 degree revolution. While the piston is at T.D.C. the compressed air-fuel mixture is ignited by a spark plug or by heat generated by high compression, forcefully returning the piston to B.D.C. This stroke produces mechanical work from the engine to turn the crankshaft.
- Exhaust: Also known as outlet. During the exhaust stroke, the piston, once again, returns from B.D.C. to T.D.C. while the exhaust valve is open. This action expels the spent air-fuel mixture through the exhaust port.

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.

Fuel efficiency is a form of thermal efficiency, meaning the ratio of effort to result of a process that converts chemical potential energy contained in a carrier (fuel) into kinetic energy or work. Overall fuel efficiency may vary per device, which in turn may vary per application, and this spectrum of variance is often illustrated as a continuous energy profile. Non-transportation applications, such as industry, benefit from increased fuel efficiency, especially fossil fuel power plants or industries dealing with combustion, such as ammonia production during the Haber process.

The Brayton cycle, also known as the Joule cycle, is a thermodynamic cycle that describes the operation of certain heat engines that have air or some other gas as their working fluid. It is characterized by isentropic compression and expansion, and isobaric heat addition and rejection, though practical engines have adiabatic rather than isentropic steps.

A combined cycle power plant is an assembly of heat engines that work in tandem from the same source of heat, converting it into mechanical energy. On land, when used to make electricity the most common type is called a combined cycle gas turbine (CCGT) plant, which is a kind of gas-fired power plant. The same principle is also used for marine propulsion, where it is called a combined gas and steam (COGAS) plant. Combining two or more thermodynamic cycles improves overall efficiency, which reduces fuel costs.
Cryogenic fuels are fuels that require storage at extremely low temperatures in order to maintain them in a liquid state. These fuels are used in machinery that operates in space where ordinary fuel cannot be used, due to the very low temperatures often encountered in space, and the absence of an environment that supports combustion. Cryogenic fuels most often constitute liquefied gases such as liquid hydrogen.
The heating value of a substance, usually a fuel or food, is the amount of heat released during the combustion of a specified amount of it.
Micro combined heat and power, micro-CHP, μCHP or mCHP is an extension of the idea of cogeneration to the single/multi family home or small office building in the range of up to 50 kW. Usual technologies for the production of heat and power in one common process are e.g. internal combustion engines, micro gas turbines, stirling engines or fuel cells.
Homogeneous Charge Compression Ignition (HCCI) is a form of internal combustion in which well-mixed fuel and oxidizer are compressed to the point of auto-ignition. As in other forms of combustion, this exothermic reaction produces heat that can be transformed into work in a heat engine.
Gasoline gallon equivalent (GGE) or gasoline-equivalent gallon (GEG) is the amount of an alternative fuel it takes to equal the energy content of one liquid gallon of gasoline. GGE allows consumers to compare the energy content of competing fuels against a commonly known fuel, namely gasoline.

A gas engine is an internal combustion engine that runs on a fuel gas, such as coal gas, producer gas, biogas, landfill gas, natural gas or hydrogen. In the United Kingdom and British English-speaking countries, the term is unambiguous. In the United States, due to the widespread use of "gas" as an abbreviation for gasoline (petrol), such an engine is sometimes called by a clarifying term, such as gaseous-fueled engine or natural gas engine.

In thermodynamics, the thermal efficiency is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.

A hydrogen internal combustion engine vehicle (HICEV) is a type of hydrogen vehicle using an internal combustion engine. Hydrogen internal combustion engine vehicles are different from hydrogen fuel cell vehicles. Instead, the hydrogen internal combustion engine is simply a modified version of the traditional gasoline-powered internal combustion engine. The absence of carbon means that no CO2 is produced, which eliminates the main greenhouse gas emission of a conventional petroleum engine.
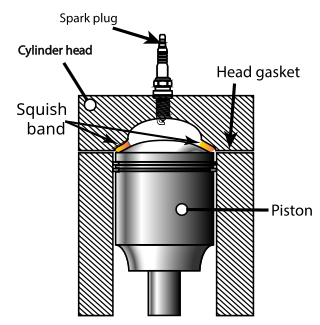
Squish is an effect in internal combustion engines which creates sudden turbulence of the air-fuel mixture as the piston approaches top dead centre (TDC).

An internal combustion engine is a heat engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons, turbine blades, a rotor, or a nozzle. This force moves the component over a distance. This process transforms chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to.
References
- ↑ "Types of Efficiencies". Combustion Technology. Retrieved 2024-08-05.
- ↑ "Combustion Efficiency Explained". saVRee. 2020-10-29. Retrieved 2024-08-05.