Related Research Articles
Evidence-based medicine (EBM) is "the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients." The aim of EBM is to integrate the experience of the clinician, the values of the patient, and the best available scientific information to guide decision-making about clinical management. The term was originally used to describe an approach to teaching the practice of medicine and improving decisions by individual physicians about individual patients.

An HIV vaccine could be either a preventive vaccine or a therapeutic vaccine, which means it will either protect individuals from being infected with HIV or treat HIV-infected individuals. And it could either induce an immune response against HIV or consist of preformed antibodies against HIV.
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs in an attempt to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same sex and opposite sex partners so long as the HIV-positive partner maintains an undetectable viral load.
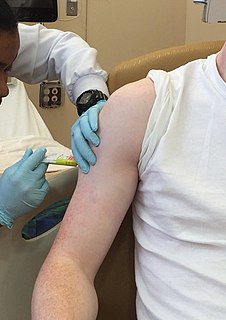
Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.
Orthomolecular medicine is a form of alternative medicine that aims to maintain human health through nutritional supplementation. The concept builds on the idea of an optimal nutritional environment in the body and suggests that diseases reflect deficiencies in this environment. Treatment for disease, according to this view, involves attempts to correct "imbalances or deficiencies based on individual biochemistry" by use of substances such as vitamins, minerals, amino acids, trace elements and fatty acids. The notions behind orthomolecular medicine are not supported by sound medical evidence, and the therapy is not effective; even the validity of calling the orthomolecular approach a form of medicine has been questioned since the 1970s.

Telehealth is the distribution of health-related services and information via electronic information and telecommunication technologies. It allows long-distance patient and clinician contact, care, advice, reminders, education, intervention, monitoring, and remote admissions. Telemedicine is sometimes used as a synonym, or is used in a more limited sense to describe remote clinical services, such as diagnosis and monitoring. When rural settings, lack of transport, a lack of mobility, decreased funding, or a lack of staff restrict access to care, telehealth may bridge the gap. as well as provider distance-learning; meetings, supervision, and presentations between practitioners; online information and health data management and healthcare system integration. Telehealth could include two clinicians discussing a case over video conference; a robotic surgery occurring through remote access; physical therapy done via digital monitoring instruments, live feed and application combinations; tests being forwarded between facilities for interpretation by a higher specialist; home monitoring through continuous sending of patient health data; client to practitioner online conference; or even videophone interpretation during a consult.
eHealth is a relatively recent healthcare practice supported by electronic processes and communication, dating back to at least 1999. Usage of the term varies as it just not covers the "Internet medicine" as it was conceived during that time, but also covers "virtually everything related to computers and medicine". A study in 2005 found 51 unique definitions. Some argue that it is interchangeable with health informatics with a broad definition covering electronic/digital processes in health while others use it in the narrower sense of healthcare practice using the Internet. It can also include health applications and links on mobile phones, referred to as mHealth or m-Health.
Matthias Rath is a controversial doctor, businessman, and vitamin salesman. He earned his medical degree in Germany. Rath claims that a program of nutritional supplements, including formulations that he sells, can treat or cure diabetes, cardiovascular disease, cancer, and HIV/AIDS. These claims are not supported by any reliable medical research. Rath runs the Dr. Rath Health Foundation, has been closely associated with Health Now, Inc., and founded the Dr. Rath Research Institute.

Ben Michael Goldacre is a British physician, academic, and science writer. As of March 2015, he is a senior clinical research fellow at the Centre for Evidence-Based Medicine, part of the University of Oxford's Nuffield Department of Primary Care Health Sciences. He is a founder of the AllTrials campaign and OpenTrials to require open science practices in clinical trials.
A clinical decision support system (CDSS) is a health information technology system that is designed to provide physicians and other health professionals with clinical decision support (CDS), that is, assistance with clinical decision-making tasks. A working definition has been proposed by Robert Hayward of the Centre for Health Evidence: "Clinical decision support systems link health observations with health knowledge to influence health choices by clinicians for improved health care". CDSSs constitute a major topic in artificial intelligence in medicine.
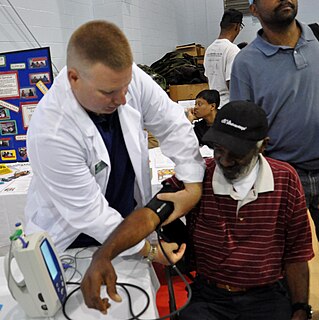
Homeless dumping or patient dumping is the practice of hospitals and emergency services inappropriately releasing homeless or indigent patients to public hospitals or on the streets instead of placing them with a homeless shelter or retaining them, especially when they may require expensive medical care with minimal government reimbursement from Medicaid or Medicare. The term homeless dumping has been used since the late 19th century and resurfaced throughout the 20th century alongside legislation and policy changes aimed at addressing the issue. Studies of the issue have indicated mixed results from the United States' policy interventions and have proposed varying ideas to remedy the problem.
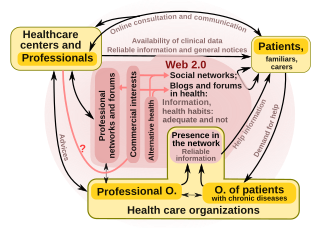
"Health 2.0" is a term introduced in the mid-2000s, as the subset of health care technologies mirroring the wider Web 2.0 movement. It has been defined variously as including social media, user-generated content, and cloud-based and mobile technologies. Some Health 2.0 proponents see these technologies as empowering patients to have greater control over their own health care and diminishing medical paternalism. Critics of the technologies have expressed concerns about possible misinformation and violations of patient privacy.

PatientsLikeMe is the world’s largest integrated community, health management, and real-world data platform. Through PatientsLikeMe, a growing community of more than 830,000 people with over 2,900 conditions share personal stories and information about their health, symptoms, and treatments, with a goal to improve the lives of all patients through knowledge derived from shared real-world experiences and outcomes. Data generated by patients themselves are systemically collected and quantified while also providing an environment for peer support and learning. These data capture the complex temporality and competing influences of different lifestyle choices, socio-demographics, conditions, and treatments on a person’s health. Everything members share empowers the community with personal agency, establishing PLM as a clinically robust resource with demonstrated impact, including more than 100 studies in peer-reviewed medical and scientific journals.
ClinicalTrials.gov is a registry of clinical trials. It is run by the United States National Library of Medicine (NLM) at the National Institutes of Health, and is the largest clinical trials database, holding registrations from over 329,000 trials from 209 countries.
Patient participation is a trend that arose in answer to perceived physician paternalism. However, only rarely can unchecked physician paternalism be justified, and unlimited patient autonomy would presumably be equally abhorrent.

Shared decision-making in medicine (SDM) is a process in which both the patient and physician contribute to the medical decision-making process and agree on treatment decisions. Health care providers explain treatments and alternatives to patients and help them choose the treatment option that best aligns with their preferences as well as their unique cultural and personal beliefs.
Michael Stuart Gottlieb is an American physician and immunologist known for his 1981 identification of acquired immune deficiency syndrome (AIDS) as a new disease, and for his HIV/AIDS research, HIV/AIDS activism, and philanthropic efforts associated with HIV/AIDS treatment.
HIV prevention refers to practices that aim to prevent the spread of the Human Immunodeficiency Virus (HIV). HIV prevention practices may be undertaken by individuals to protect their own health and the health of those in their community, or may be instituted by governments and community-based organizations as public health policies.

HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
Julio S. G. Montaner, is an Argentine-Canadian physician, professor and researcher. He is the director of the British Columbia Centre for Excellence in HIV/AIDS, the chair in AIDS Research and head of the Division of AIDS in the Faculty of Medicine at the University of British Columbia and the past-president of the International AIDS Society. He is also the director of the John Ruedy Immunodeficiency Clinic, and the Physician Program Director for HIV/AIDS PHC. He is known for his work on HAART, a role in the discovery of triple therapy as an effective treatment for HIV in the late 1990s, and a role in advocating the "Treatment as Prevention" Strategy in the mid-2000s, led by Myron Cohen of the HPTN 052 trial.
References
- ↑ University of Texas Medical Branch, AIDS Clinical Trials Unit, "History of CABs"
- ↑ Phyllis Maguire (July–August 1999). "Community-based trials under scrutiny". ACP-ASIM Observer. Archived from the original on 2007-05-26. Retrieved 2007-03-07.
- ↑ Silversides A (January 2004). "The tribulations of community-based trials". CMAJ. 170 (1): 33. PMC 305306 . PMID 14707212.