

Conium alkaloids are natural products of the piperidine alkaloid type.


Conium alkaloids are natural products of the piperidine alkaloid type.
Conium alkaloids are found in spotted hemlock. The mature fruits may contain up to 3.5% alkaloids. [1]
The main alkaloid is coniine. Other representatives are γ-conicein, conhydrin, pseudoconhydrin, and N-methylconiin. [1]
Most Conium alkaloids are liquid at room temperature. [2]
500 mg of coniin is fatal to a human. [1] Coniin is the poison of the spotted hemlock. Poisoning results in nausea, vomiting, salivation, and diarrhea. Within half an hour to an hour, paralysis of the chest muscles occurs, which is fatal. [3] [4]
In ancient times, aqueous extracts of this plant ( hemlock cup ) were administered. [4] In 399 BC, Socrates was sentenced to death by the cup of hemlock as a "free thinker and seducer of youth." [5]

Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. More rarely still, they may contain elements such as phosphorus, chlorine, and bromine.

Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, typical of amines. The name comes from the genus name Piper, which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins.

Conium is a genus of flowering plants in the family Apiaceae. As of December 2020, Plants of the World Online accepts six species.

Coniine is a poisonous chemical compound, an alkaloid present in and isolable from poison hemlock (Conium maculatum), where its presence has been a source of significant economic, medical, and historico-cultural interest; coniine is also produced by the yellow pitcher plant (Sarracenia flava), and fool's parsley (Aethusa cynapium). Its ingestion and extended exposure are toxic to humans and all classes of livestock; its mechanism of poisoning involves disruption of the central nervous system, with death caused by respiratory paralysis. The biosynthesis of coniine contains as its penultimate step the non-enzymatic cyclisation of 5-oxooctylamine to γ-coniceine, a Schiff base differing from coniine only by its carbon-nitrogen double bond in the ring. This pathway results in natural coniine that is a mixture—a racemate—composed of two enantiomers, the stereoisomers (S)-(+)-coniine and (R)-(−)-coniine, depending on the direction taken by the chain that branches from the ring. Both enantiomers are toxic, with the (R)-enantiomer being the more biologically active and toxic of the two in general. Coniine holds a place in organic chemistry history as being the first of the important class of alkaloids to be synthesized, by Albert Ladenburg in 1886, and it has been synthesized in the laboratory in a number of unique ways through to modern times.
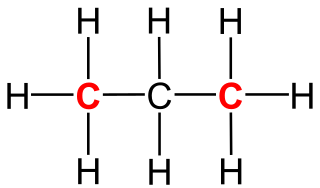
In organic chemistry, a primary carbon is a carbon atom which is bound to only one other carbon atom. It is thus at the end of a carbon chain. In case of an alkane, three hydrogen atoms are bound to a primary carbon. A hydrogen atom could also be replaced by a hydroxy group, which would make the molecule a primary alcohol.

A secondary carbon is a carbon atom bound to two other carbon atoms. For this reason, secondary carbon atoms are found in almost all hydrocarbons having at least three carbon atoms. In unbranched alkanes, the inner carbon atoms are always secondary carbon atoms.

The hemlock moth, also known as the defoliating hemlock moth or poison hemlock moth, is a nocturnal moth species of the family Depressariidae. Of Palaearctic origin, it was first found in North America in 1973 when it was accidentally introduced. The moth is now widespread throughout the northern half of the United States, southern Canada, northern Europe, and, more recently, New Zealand and Australia. The larval form grows to around 10 mm, while the adults wingspan is between 17 mm and 19 mm.

Phenolates are anions, salts, and esters of phenols, containing the phenolate ion. They may be formed by reaction of phenols with strong base.

Conium maculatum, colloquially known as hemlock, poison hemlock or wild hemlock, is a highly poisonous flowering plant in the carrot family Apiaceae, native to Europe and North Africa. It is herbaceous without woody parts and has a biennial lifecycle. A hardy plant capable of living in a variety of environments, hemlock is widely naturalised in locations outside its native range, such as parts of Australia, West Asia, and North and South America, to which it has been introduced. It is capable of spreading and thereby becoming an invasive weed.

Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.

Steroidal alkaloids have the basic steroidal skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic cyclopentanoperhydrophenanthrene skeleton that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in Chonemorpha fragrans, 'chonemorphine' was used to treat intestinal infections in Wistar rats..
Streptomyces chrestomyceticus is a bacterium species from the genus of Streptomyces. Streptomyces chrestomyceticus produces lycopene, pyrrolostatin, paromomycin, aminocidin, aminosidin, neomycin E and neomycin F.
David Rytz von Brugg was a Swiss mathematician and teacher.

The Bailey peptide synthesis is a name reaction in organic chemistry developed 1949 by J. L. Bailey. It is a method for the synthesis of a peptide from α-amino acid-N-carboxylic acid anhydrides (NCAs) and amino acids or peptide esters. The reaction is characterized by short reaction times and a high yield of the target peptide.
Polyvinyl esters are a group of thermoplastic vinyl polymers. The most important examples of this group are polyvinyl acetate (PVAC) and polyvinyl propionate.

Isoquinoline alkaloids are natural products of the group of alkaloids, which are chemically derived from isoquinoline. They form the largest group among the alkaloids.
Bisbenzylisoquinoline alkaloids are natural products found primarily in the plant families of the barberry family, the Menispermaceae, the Monimiaceae, and the buttercup family.

Indolizidine alkaloids are natural products from various alkaloid groups whose structure can be derived from indolizidine.

The pyrrolidine alkaloids are natural products chemically derived from pyrrolidine.

Strychnos alkaloids are natural products primarily found in the seeds of the strychnine tree and in the genus catharanthus.