
The United States Food and Drug Administration is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products.

Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) and strengths of final products.

A prescription drug is a pharmaceutical drug that is permitted to be dispensed only to those with a medical prescription. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practicing medicine without a license and without sufficient education. Different jurisdictions have different definitions of what constitutes a prescription drug.

Current good manufacturing practices (cGMP) are those conforming to the guidelines recommended by relevant agencies. Those agencies control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines provide minimum requirements that a manufacturer must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use.
The United States Pharmacopeia (USP) is a pharmacopeia for the United States published annually by the over 200-year old United States Pharmacopeial Convention, a nonprofit organization that owns the trademark and also owns the copyright on the pharmacopeia itself.

The Natural Products Association or NPA is the largest and oldest nonprofit organization representing the interests of manufacturers and retailers of the natural products industry, which includes organic and health foods, dietary supplements, natural ingredient cosmetics, and other similar products. The organization includes more than 1,900 members accounting for more than 10,000 retailers, manufacturers, wholesalers and distributors of natural products.
The pharmaceutical industry is one of the leading industries in the People's Republic of China, covering synthetic chemicals and drugs, prepared Chinese medicines, medical devices, apparatus and instruments, hygiene materials, packing materials, and pharmaceutical machinery. China has the second-largest pharmaceutical market in the world as of 2017 which is worth US$110 billion. China accounts for 20% of the world's population but only a small fraction of the global drug market. China's changing health-care environment is designed to extend basic health insurance to a larger portion of the population and give individuals greater access to products and services. Following the period of change, the pharmaceutical industry is expected to continue its expansion.

Perrigo Company plc is an American Irish-registered manufacturer of private label over-the-counter pharmaceuticals, and while 70% of Perrigo's net sales are from the U.S. healthcare system, Perrigo is legally headquartered in Ireland for tax purposes, which accounts for 0.60% of net sales. In 2013, Perrigo completed the sixth-largest US corporate tax inversion in history when it reregistered its tax status to Ireland to avoid U.S. corporate taxes. Perrigo maintains its corporate headquarters in Grand Rapids, Michigan, within Michigan State University's Grand Rapids Innovation Park.
Amerifit Brands, also commonly known as Amerifit, Inc. and Amerifit Nutrition, is an American corporation that produces numerous health and wellness brands, such as Estroven, AZO, and Culturelle. Its headquarters are located in Cromwell, Connecticut, but were previously located in Bloomfield, nearly 25 miles away. Its current CEO is Wes Parris.
Title 21 is the portion of the Code of Federal Regulations that governs food and drugs within the United States for the Food and Drug Administration (FDA), the Drug Enforcement Administration (DEA), and the Office of National Drug Control Policy (ONDCP).
Prestige Consumer Healthcare Inc. is an American company that markets and distributes over-the-counter healthcare and household cleaning products. It was formed by the merger of Medtech Products, Inc., Prestige Brands International, and the Spic and Span Company in 1996. The company is headquartered in Tarrytown, New York and operates a manufacturing facility in Lynchburg, Virginia.
McNeil Consumer Healthcare is an American medicals products company belonging to Kenvue consumer health group. It primarily sells fast-moving consumer goods such as over-the-counter drugs.
Structured Product Labeling (SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug. As of Release 4 of the SPL standard, 22,000 FDA informational product inserts have been encoded according to the standard.
The Center for Food Safety and Applied Nutrition is the branch of the United States Food and Drug Administration (FDA) that regulates food, dietary supplements, and cosmetics, as opposed to drugs, biologics, medical devices, and radiological products, which also fall under the purview of the FDA.
Dexatrim is an over-the-counter (OTC) dietary supplement meant to assist with weight loss. Dexatrim claims it "gives you the power to lose weight, curb binges, and keep you in control of your diet." Current Dexatrim products available are in capsule form and include Dexatrim Max Complex 7, Dexatrim Max Daytime Appetite Control, Dexatrim Natural Green Tea, and Dexatrim Natural Extra Energy. The major active ingredients found in current Dexatrim products include caffeine, green tea extract, Asian (Panax) ginseng root extract, and dehydroepiandrosterone (DHEA).
Over-the-counter counseling refers to the counseling that a pharmacist may provide on the subject of initiating, modifying, or stopping an over-the-counter (OTC) drug product. OTC counseling requires an assessment of the patient's self-care concerns and drug-related needs. The types of drugs that are involved in OTC counseling are, for example, used to treat self-diagnosable conditions like heartburn, cough, and rashes, though prescription drugs and professional diagnoses are also relevant to the recommendation process.

China Nepstar Chain Drugstore Ltd., which conducts business as China Nepstar, is China's largest retail drugstore chain, based on the number of directly operated stores. It is headquartered in Nanshan District, Shenzhen.
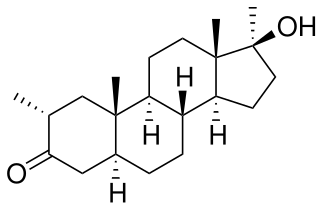
Methasterone, also known as methyldrostanolone and known by the nickname Superdrol, is a synthetic and orally active anabolic–androgenic steroid (AAS) which was never marketed for medical use. It was sold legally for 9 years as a body building supplement. Because of this lengthy time being legal it has more studies and references than most other designer steroids.
Crookes Healthcare is a healthcare manufacturer based in Nottingham, England, and a subsidiary of Reckitt. It manufactures some of the best-known health remedies and over-the-counter drugs sold by British pharmacies.
The Council for Responsible Nutrition (CRN) is a Washington D.C.-based trade association and lobbying group representing more than 180 companies that manufacture dietary ingredients and supplements, or supply services to those suppliers and manufacturers. CRN's current president and CEO is Steve M. Mister.








