
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.
In chemistry, electron counting is a formalism for assigning a number of valence electrons to individual atoms in a molecule. It is used for classifying compounds and for explaining or predicting their electronic structure and bonding. Many rules in chemistry rely on electron-counting:

In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".

A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal ions to ligands involves this kind of interaction. This type of interaction is central to Lewis acid–base theory.
A substitution reaction is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent.
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination.

In chemistry, a formal charge, in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. In simple terms, formal charge is the difference between the number of valence electrons of an atom in a neutral free state and the number assigned to that atom in a Lewis structure. When determining the best Lewis structure for a molecule, the structure is chosen such that the formal charge on each of the atoms is as close to zero as possible.

Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy.
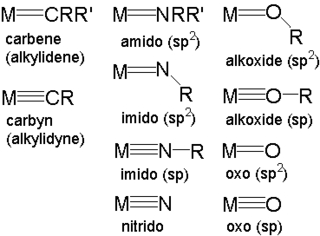
In organometallic chemistry, a metal–ligand multiple bond describes the interaction of certain ligands with a metal with a bond order greater than one. Coordination complexes featuring multiply bonded ligands are of both scholarly and practical interest. Transition metal carbene complexes catalyze the olefin metathesis reaction. Metal oxo intermediates are pervasive in oxidation catalysis.
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal, an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition.
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; crystal field theory and ligand field theory, which is a more advanced version based on molecular orbital theory. However the d electron count of an atom in a complex is often different from the d electron count of a free atom or a free ion of the same element.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.
Ligand bond number (LBN) represents the effective total number of ligands or ligand attachment points surrounding a metal center, labeled M. More simply, it represents the number of coordination sites occupied on the metal. Based on the covalent bond classification method, the equation for determining ligand bond number is as follows:
In covalent bond classification, a Z-type ligand refers to a ligand that accepts two electrons from the metal center. This is in contrast to X-type ligands, which form a bond with the ligand and metal center each donating one electron, and L-type ligands, which form a bond with the ligand donating two electrons. Typically, these Z-type ligands are Lewis acids, or electron acceptors. They are also known as zero-electron reagents.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.

Alkaline earth octacarbonyl complexes are a class of neutral compounds that have the general formula M(CO)8 where M is a heavy Group 2 element (Ca, Sr, or Ba). The metal center has a formal oxidation state of 0 and the complex has a high level of symmetry belonging to the cubic Oh point group. These complexes are isolable in a low-temperature neon matrix, but are not frequently used in applications due to their instability in air and water. The bonding within these complexes is controversial with some arguing the bonding resembles a model similar to bonding in transition metal carbonyl complexes which abide by the 18-electron rule, and others arguing the molecule more accurately contains ionic bonds between the alkaline earth metal center and the carbonyl ligands. Complexes of Be(CO)8 and Mg(CO)8 are not synthetically possible due to inaccessible (n-1)d orbitals. Beryllium has been found to form a dinuclear homoleptic carbonyl and magnesium a mononuclear heteroleptic carbonyl, both with only two carbonyl ligands instead of eight to each metal atom.

Organoberyllium chemistry involves the synthesis and properties of organometallic compounds featuring the group 2 alkaline earth metal beryllium (Be). The area remains understudied, relative to the chemistry of other main-group elements, because although metallic beryllium is relatively unreactive, its dust causes berylliosis and compounds are toxic. Organoberyllium compounds are typically prepared by transmetallation or alkylation of beryllium chloride.

















