A cyanostar (pentacyanopentabenzo[25]annulene) is a shape-persistent macrocycle that binds anions. [1] [2]
Contents
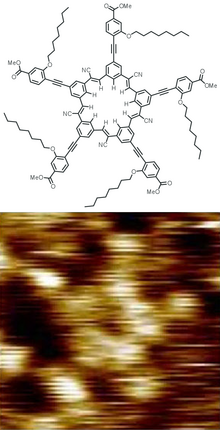
A cyanostar (pentacyanopentabenzo[25]annulene) is a shape-persistent macrocycle that binds anions. [1] [2]

The cyanostar structure is synthesized in a one-pot process among five equivalents of a benzaldehyde bearing a meta -cyanomethyl substituent. A series of Knoevenagel condensation reactions catalyzed by various bases stitches them together to make the C5-symmetric structure. [3]
Cyanostar binds anions through hydrogen bonding from the C–H bonds, as the hydrogen has a positive electrostatic potential. [3] It is the first binder to make use of cyanostilbene's electropositive CH groups. The CH bonds create an electropositive region in the center of the macrocycle, creating a binding pocket. Cyanostar strongly binds anions that usually can only be bound weakly. The increased binding arises from the formation of a 2:1 complex, with two cyanostars sandwiching the anion on each side. [3] An extended version of this structural pattern is a 4:3 alternating stack of cyanostar molecules complexing a hydrogen-bonded chain of dihydrogen phosphate units. [4]
Two cyanostars can be threaded onto a phosphate diester structure, forming a rotaxane. Because they have a high affinity for the central phosphate group only when it is in its anionic form, there is a substantial and reversible structural change in response to acid–base changes in solution. [5]

In chemistry, a hydrogen bond is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn−H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the period 2 elements nitrogen (N), oxygen (O), and fluorine (F).

A rotaxane is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle. The two components of a rotaxane are kinetically trapped since the ends of the dumbbell are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds.

Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
A polycatenane is a chemical substance that, like polymers, is chemically constituted by a large number of units. These units are made up of concatenated rings into a chain-like structure.
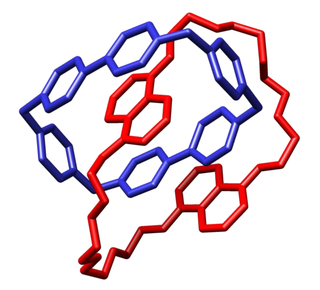
In macromolecular chemistry, a catenane is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis.

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another.

In host-guest chemistry, cucurbiturils are macrocyclic molecules made of glycoluril monomers linked by methylene bridges. The oxygen atoms are located along the edges of the band and are tilted inwards, forming a partly enclosed cavity (cavitand). The name is derived from the resemblance of this molecule with a pumpkin of the family of Cucurbitaceae.

Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. The reaction is related to olefin metathesis.
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/mol. Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects.

In chemistry, a foldamer is a discrete chain molecule (oligomer) that folds into a conformationally ordered state in solution. They are artificial molecules that mimic the ability of proteins, nucleic acids, and polysaccharides to fold into well-defined conformations, such as α-helices and β-sheets. The structure of a foldamer is stabilized by noncovalent interactions between nonadjacent monomers. Foldamers are studied with the main goal of designing large molecules with predictable structures. The study of foldamers is related to the themes of molecular self-assembly, molecular recognition, and host–guest chemistry.
In chemistry, mechanically interlocked molecular architectures (MIMAs) are molecules that are connected as a consequence of their topology. This connection of molecules is analogous to keys on a keychain loop. The keys are not directly connected to the keychain loop but they cannot be separated without breaking the loop. On the molecular level, the interlocked molecules cannot be separated without the breaking of the covalent bonds that comprise the conjoined molecules; this is referred to as a mechanical bond. Examples of mechanically interlocked molecular architectures include catenanes, rotaxanes, molecular knots, and molecular Borromean rings. Work in this area was recognized with the 2016 Nobel Prize in Chemistry to Bernard L. Feringa, Jean-Pierre Sauvage, and J. Fraser Stoddart.

Cation–π interaction is a noncovalent molecular interaction between the face of an electron-rich π system (e.g. benzene, ethylene, acetylene) and an adjacent cation (e.g. Li+, Na+). This interaction is an example of noncovalent bonding between a monopole (cation) and a quadrupole (π system). Bonding energies are significant, with solution-phase values falling within the same order of magnitude as hydrogen bonds and salt bridges. Similar to these other non-covalent bonds, cation–π interactions play an important role in nature, particularly in protein structure, molecular recognition and enzyme catalysis. The effect has also been observed and put to use in synthetic systems.

The Hofmeister series or lyotropic series is a classification of ions in order of their lyotrophic properties, which is the ability to salt out or salt in proteins. The effects of these changes were first worked out by Franz Hofmeister, who studied the effects of cations and anions on the solubility of proteins.
Cyclodiphosphazanes are saturated four membered P2N2 ring systems and one of the major classes of cyclic phosphazene compounds. Bis(chloro)cyclodiphosphazanes, (cis-[ClP(μ-NR)]2) are important starting compounds for synthesizing a variety of cyclodiphosphazane derivatives by nucleophilic substitution reactions; are prepared by reaction of phosphorus trichloride (PCl3) with a primary amine (RNH2) or amine hydrochlorides (RNH3Cl).
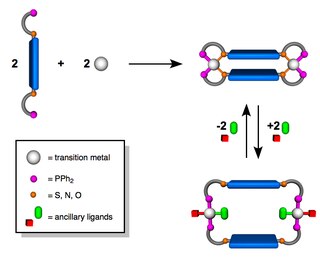
The Weak-Link Approach (WLA) is a supramolecular coordination-based assembly methodology, first introduced in 1998 by the Mirkin Group at Northwestern University. This method takes advantage of hemilabile ligands -ligands that contain both strong and weak binding moieties- that can coordinate to metal centers and quantitatively assemble into a single condensed ‘closed’ structure. Unlike other supramolecular assembly methods, the WLA allows for the synthesis of supramolecular complexes that can be modulated from rigid ‘closed’ structures to flexible ‘open’ structures through reversible binding of allosteric effectors at the structural metal centers. The approach is general and has been applied to a variety of metal centers and ligand designs including those with utility in catalysis and allosteric regulation.

Hydrogen-bond catalysis is a type of organocatalysis that relies on use of hydrogen bonding interactions to accelerate and control organic reactions. In biological systems, hydrogen bonding plays a key role in many enzymatic reactions, both in orienting the substrate molecules and lowering barriers to reaction. The field is relatively undeveloped compared to research in Lewis acid catalysis.
DNA-binding metallo-intercalators are positively charged, planar, polycyclic, aromatic compounds that unwind the DNA double helix and insert themselves between DNA base pairs. Metallo-intercalators insert themselves between two intact base pairs without expelling or replacing the original nitrogenous bases; the hydrogen bonds between the nitrogenous bases at the site of intercalation remain unbroken. In addition to π-stacking between the aromatic regions of the intercalator and the nitrogenous bases of DNA, intercalation is stabilized by van der Waals, hydrophobic, electrostatic, and entropic interactions. This ability to bind to specific DNA base pairs allows for potential therapeutic applications of metallo-intercalators.

Cyclobis(paraquat-p-phenylene) belongs to the class of cyclophanes, and consists of aromatic units connected by methylene bridges. It is able to incorporate small guest molecule and has played an important role in host–guest chemistry and supramolecular chemistry.
In chemistry, a pnictogen bond (PnB) is a non-covalent interaction, occurring where there is a net attractive force between an electrophilic region on a 'donor' pnictogen atom (Pn) in a molecule, and a nucleophilic region on an 'acceptor' atom, which may be in the same or another molecule. Closely related to halogen and chalcogen bonding, pnictogen bonds are a form of non-covalent interaction which can be considered in terms of charge-transfer and electrostatic interactions.

Metal–organic nanotubes (MONTs) are a class of crystalline coordination polymers consisting of organic ligands bonded to a metal or metal cluster that form single-walled one-dimensional porous structures. The usage of organic ligands allows the properties of the resulting material to be tuned, as in the parent class of metal-organic frameworks (MOFs), but like carbon nanotubes, MONTs are anisotropic structures.