
Inhalants are a broad range of household and industrial chemicals whose volatile vapors or pressurized gases can be concentrated and breathed in via the nose or mouth to produce intoxication, in a manner not intended by the manufacturer. They are inhaled at room temperature through volatilization or from a pressurized container, and do not include drugs that are sniffed after burning or heating. For example, amyl nitrite (poppers), nitrous oxide and toluene – a solvent widely used in contact cement, permanent markers, and certain types of glue – are considered inhalants, but smoking tobacco, cannabis, and crack cocaine are not, even though these drugs are inhaled as smoke or vapor.

A scuba set, originally just scuba, is any breathing apparatus that is entirely carried by an underwater diver and provides the diver with breathing gas at the ambient pressure. Scuba is an anacronym for self-contained underwater breathing apparatus. Although strictly speaking the scuba set is only the diving equipment that is required for providing breathing gas to the diver, general usage includes the harness or rigging by which it is carried, and those accessories which are integral parts of the harness and breathing apparatus assembly, such as a jacket or wing style buoyancy compensator and instruments mounted in a combined housing with the pressure gauge, and in the looser sense, it has been used to refer to all the diving equipment used by the scuba diver, though this would more commonly and accurately be termed scuba equipment or scuba gear. Scuba is overwhelmingly the most common underwater breathing system used by recreational divers and is also used in professional diving when it provides advantages, usually of mobility and range, over surface-supplied diving systems, and is allowed by the relevant legislation and code of practice.

Nitrous oxide, commonly known as laughing gas, nitrous, nitro, or nos, is a chemical compound, an oxide of nitrogen with the formula N
2O. At room temperature, it is a colourless non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful oxidiser similar to molecular oxygen.

A diving cylinder or diving gas cylinder is a gas cylinder used to store and transport high pressure gas used in diving operations. This may be breathing gas used with a scuba set, in which case the cylinder may also be referred to as a scuba cylinder, scuba tank or diving tank. When used for an emergency gas supply for surface supplied diving or scuba, it may be referred to as a bailout cylinder or bailout bottle. It may also be used for surface-supplied diving or as decompression gas. A diving cylinder may also be used to supply inflation gas for a dry suit or buoyancy compensator. Cylinders provide gas to the diver through the demand valve of a diving regulator or the breathing loop of a diving rebreather.
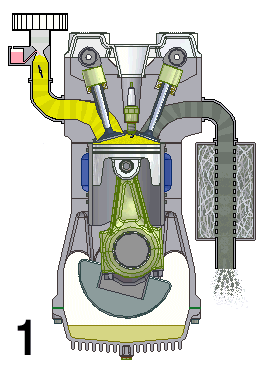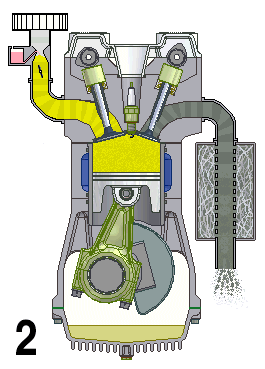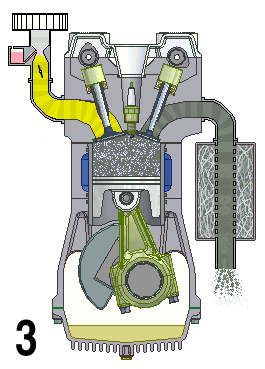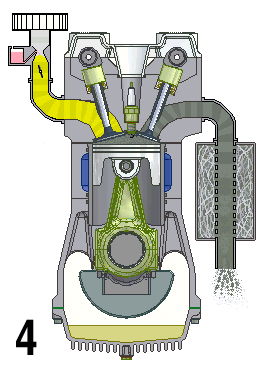
A naturally aspirated engine, also known as a normally aspirated engine, and abbreviated to N/A or NA, is an internal combustion engine in which air intake depends solely on atmospheric pressure and does not have forced induction through a turbocharger or a supercharger.
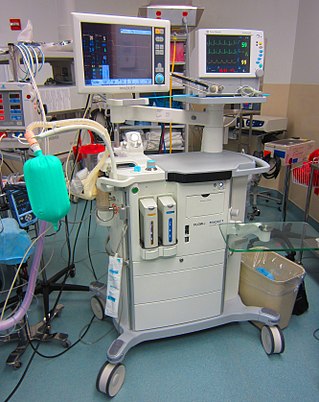
An anaesthetic machine or anesthesia machine is a medical device used to generate and mix a fresh gas flow of medical gases and inhalational anaesthetic agents for the purpose of inducing and maintaining anaesthesia.

A Nitrous Oxide Engine, or Nitrous Oxide System commonly referred to and known as NOS, is an internal combustion engine in which oxygen for burning the fuel comes from the decomposition of nitrous oxide, N2O, as well as air. The system increases the engine's power output by allowing fuel to be burned at a higher-than-normal rate, because of the higher partial pressure of oxygen injected with the fuel mixture. Nitrous injection systems may be "dry", where the nitrous oxide is injected separately from fuel, or "wet" in which additional fuel is carried into the engine along with the nitrous. NOS may not be permitted for street or highway use, depending on local regulations. N2O use is permitted in certain classes of auto racing. Reliable operation of an engine with nitrous injection requires careful attention to the strength of engine components and to the accuracy of the mixing systems, otherwise destructive detonations or exceeding engineered component maximums may occur. Nitrous oxide systems were applied as early as World War II for certain aircraft engines.

Bottled gas is a term used for substances which are gaseous at standard temperature and pressure (STP) and have been compressed and stored in carbon steel, stainless steel, aluminum, or composite containers known as gas cylinders.

Nitrous oxide is an inhaled gas used as a pain medication and together with other medications for anesthesia. Common uses include during childbirth, following trauma, and as part of end-of-life care. Onset of effect is typically within half a minute, and the effect lasts for about a minute.

A gas cylinder is a pressure vessel for storage and containment of gases at above atmospheric pressure. High-pressure gas cylinders are also called bottles. Inside the cylinder the stored contents may be in a state of compressed gas, vapor over liquid, supercritical fluid, or dissolved in a substrate material, depending on the physical characteristics of the contents. A typical gas cylinder design is elongated, standing upright on a flattened bottom end, with the valve and fitting at the top for connecting to the receiving apparatus.
An oxygen concentrator is a device that concentrates the oxygen from a gas supply by selectively removing nitrogen to supply an oxygen-enriched product gas stream. They are used industrially, to provide supplemental oxygen at high altitudes, and as medical devices for oxygen therapy.
Medical gas supply systems in hospitals and other healthcare facilities are utilized to supply specialized gases and gas mixtures to various parts of the facility. Products handled by such systems typically include:

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.

Oxy-fuel welding and oxy-fuel cutting are processes that use fuel gases and oxygen to weld or cut metals. French engineers Edmond Fouché and Charles Picard became the first to develop oxygen-acetylene welding in 1903. Pure oxygen, instead of air, is used to increase the flame temperature to allow localised melting of the workpiece material in a room environment. A common propane/air flame burns at about 2,250 K, a propane/oxygen flame burns at about 2,526 K, an oxyhydrogen flame burns at 3,073 K and an acetylene/oxygen flame burns at about 3,773 K.

Scuba gas planning is the aspect of dive planning and of gas management which deals with the calculation or estimation of the amounts and mixtures of gases to be used for a planned dive. It may assume that the dive profile, including decompression, is known, but the process may be iterative, involving changes to the dive profile as a consequence of the gas requirement calculation, or changes to the gas mixtures chosen. Use of calculated reserves based on planned dive profile and estimated gas consumption rates rather than an arbitrary pressure is sometimes referred to as rock bottom gas management. The purpose of gas planning is to ensure that for all reasonably foreseeable contingencies, the divers of a team have sufficient breathing gas to safely return to a place where more breathing gas is available. In almost all cases this will be the surface.

Scuba gas management is the aspect of scuba diving which includes the gas planning, blending, filling, analysing, marking, storage, and transportation of gas cylinders for a dive, the monitoring and switching of breathing gases during a dive, efficient and correct use of the gas, and the provision of emergency gas to another member of the dive team. The primary aim is to ensure that everyone has enough to breathe of a gas suitable for the current depth at all times, and is aware of the gas mixture in use and its effect on decompression obligations, nitrogen narcosis, and oxygen toxicity risk. Some of these functions may be delegated to others, such as the filling of cylinders, or transportation to the dive site, but others are the direct responsibility of the diver using the gas.
Gas blending is the process of mixing gases for a specific purpose where the composition of the resulting mixture is specified and controlled. A wide range of applications include scientific and industrial processes, food production and storage and breathing gases.

Nitrous oxide (street name nangs, hippy crack, whippets or whippits) is a gas which can induce euphoria, hallucinogenic states and relaxation when inhaled. When used consistently and extensively for long periods of time (generally multiple months or longer of heavy daily use), nitrous oxide, which while normally completely non-toxic can become a potential neurotoxin by causing Vitamin B12 deficiency, and as such, excessive use for extended periods, and especially if done without external B12 supplemention can cause long-term neurological damage.

Inhalation sedation is a form of conscious sedation where an inhaled drug should:
- Depress the central nervous system (CNS) to an extent that surgeons can operate with minimal physiological and psychological stress to the patient
- Modify the patient's state of mind such that communication is maintained and the patient can respond to verbal command
- Carry a margin of safety wide enough to render the unintended loss of consciousness and loss of protective reflexes unlikely.















