Related Research Articles

Gene therapy is a medical technology that aims to produce a therapeutic effect through the manipulation of gene expression or through altering the biological properties of living cells.

Hearing loss is a partial or total inability to hear. Hearing loss may be present at birth or acquired at any time afterwards. Hearing loss may occur in one or both ears. In children, hearing problems can affect the ability to acquire spoken language, and in adults it can create difficulties with social interaction and at work. Hearing loss can be temporary or permanent. Hearing loss related to age usually affects both ears and is due to cochlear hair cell loss. In some people, particularly older people, hearing loss can result in loneliness.
Tinnitus is a variety of sound that is heard when no corresponding external sound is present. Nearly everyone experiences faint "normal tinnitus" in a completely quiet room; but it is of concern only if it is bothersome, interferes with normal hearing, or is associated with other problems. The word tinnitus comes from the Latin tinnire, "to ring". In some people, it interferes with concentration, and can be associated with anxiety and depression.
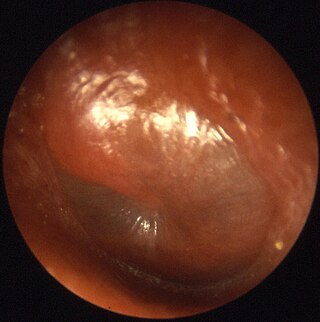
Otitis media is a group of inflammatory diseases of the middle ear. One of the two main types is acute otitis media (AOM), an infection of rapid onset that usually presents with ear pain. In young children this may result in pulling at the ear, increased crying, and poor sleep. Decreased eating and a fever may also be present. The other main type is otitis media with effusion (OME), typically not associated with symptoms, although occasionally a feeling of fullness is described; it is defined as the presence of non-infectious fluid in the middle ear which may persist for weeks or months often after an episode of acute otitis media. Chronic suppurative otitis media (CSOM) is middle ear inflammation that results in a perforated tympanic membrane with discharge from the ear for more than six weeks. It may be a complication of acute otitis media. Pain is rarely present. All three types of otitis media may be associated with hearing loss. If children with hearing loss due to OME do not learn sign language, it may affect their ability to learn.
Ototoxicity is the property of being toxic to the ear (oto-), specifically the cochlea or auditory nerve and sometimes the vestibular system, for example, as a side effect of a drug. The effects of ototoxicity can be reversible and temporary, or irreversible and permanent. It has been recognized since the 19th century. There are many well-known ototoxic drugs used in clinical situations, and they are prescribed, despite the risk of hearing disorders, for very serious health conditions. Ototoxic drugs include antibiotics, loop diuretics, and platinum-based chemotherapy agents. A number of nonsteroidal anti-inflammatory drugs (NSAIDS) have also been shown to be ototoxic. This can result in sensorineural hearing loss, dysequilibrium, or both. Some environmental and occupational chemicals have also been shown to affect the auditory system and interact with noise.
Hyperacusis is an increased sensitivity to sound and a low tolerance for environmental noise. Definitions of hyperacusis can vary significantly, but it is often categorized into four subtypes: loudness, pain, annoyance, and fear. It can be a highly debilitating hearing disorder.
Presbycusis, or age-related hearing loss, is the cumulative effect of aging on hearing. It is a progressive and irreversible bilateral symmetrical age-related sensorineural hearing loss resulting from degeneration of the cochlea or associated structures of the inner ear or auditory nerves. The hearing loss is most marked at higher frequencies. Hearing loss that accumulates with age but is caused by factors other than normal aging is not presbycusis, although differentiating the individual effects of distinct causes of hearing loss can be difficult.

George D. Yancopoulos is a Greek-American biomedical scientist who is the co-founder, president and chief scientific officer of Regeneron Pharmaceuticals.

Regeneron Pharmaceuticals, Inc. is an American biotechnology company headquartered in Westchester County, New York. The company was founded in 1988. Originally focused on neurotrophic factors and their regenerative capabilities, giving rise to its name, the company then branched out into the study of both cytokine and tyrosine kinase receptors, which gave rise to their first product, which is a VEGF-trap.

Branchio-oto-renal syndrome (BOR) is an autosomal dominant genetic disorder involving the kidneys, ears, and neck. It is also known as Melnick-Fraser syndrome.

Otoferlin is a protein that in humans is encoded by the OTOF gene. It is involved in vesicle membrane fusion, and mutations in the OTOF gene are associated with a genetic form of deafness.
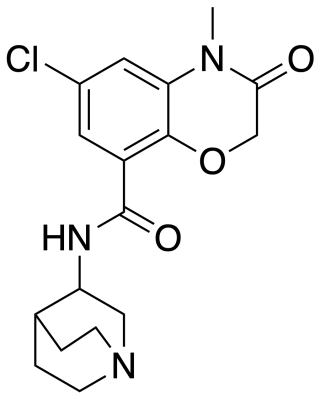
Azasetron is an antiemetic which acts as a 5-HT3 receptor antagonist, pKi = 9.27 It is used in the management of nausea and vomiting induced by cancer chemotherapy (such as cisplatin chemotherapy). Azasetron hydrochloride is given in a usual dose of 10 mg once daily by mouth or intravenously. It is approved for marketing in Japan, and marketed exclusively by Torii Pharmaceutical Co., Ltd. under the trade names "Serotone I.V. Injection 10 mg" and "Serotone Tablets 10 mg". Pharmacokinetics data from S. Tsukagoshi.
Evolocumab, sold under the brand name Repatha, is a monoclonal antibody that is an immunotherapy medication for the treatment of hyperlipidemia.

Intellia Therapeutics, Inc. is an American clinical-stage biotechnology company focused on developing novel, potentially curative therapeutics leveraging CRISPR-based technologies. The company's in vivo programs use intravenously administered CRISPR as the therapy, in which the company's proprietary delivery technology enables highly precise editing of disease-causing genes directly within specific target tissues. Intellia's ex vivo programs use CRISPR to create the therapy by using engineered human cells to treat cancer and autoimmune diseases.

Casirivimab/imdevimab, sold under the brand name REGEN‑COV among others, is a combination medicine used for the treatment and prevention of COVID‑19. It consists of two human monoclonal antibodies, casirivimab and imdevimab that must be mixed together and administered as an infusion or subcutaneous injection. The combination of two antibodies is intended to prevent mutational escape. It is also available as a co-formulated product. It was developed by the American biotechnology company Regeneron Pharmaceuticals.
Atoltivimab/maftivimab/odesivimab, sold under the brand name Inmazeb, is a fixed-dose combination of three monoclonal antibodies for the treatment of Zaire ebolavirus. It contains atoltivimab, maftivimab, and odesivimab-ebgn and was developed by Regeneron Pharmaceuticals.

Ultragenyx is an American biopharmaceutical company involved in the research and development of novel products for treatment of rare and ultra-rare genetic diseases for which there are typically no approved treatments and high unmet medical need. The company works with multiple drug modalities including biologics, small molecule, gene therapies, and ASO and mRNAs in the disease categories of bone, endocrine, metabolic, muscle and CNS diseases.
VERVE-101 and VERVE-102 are an experimental gene therapy developed by Verve Therapeutics that targets the PCSK9 gene and is intended to reduce blood cholesterol levels. It works on the same protein as the cholesterol-lowering drugs known as PCSK9 inhibitors but, unlike them, is permanent. It works via base editing, a form of CRISPR gene editing. It is one of the first gene therapies that could be beneficial to a wider segment of the population, in contrast to earlier gene therapies that were developed to treat a rare genetic disorder. Both treatments use the same RNA gene editing technology, but they use a different lipid nanoparticle delivery vehicle.
AAVAnc80-hOTOF is an experimental gene therapy for otoferlin gene-mediated hearing loss.
TSHA-102 is an experimental gene therapy developed by Taysha Gene Therapies for Rett syndrome, delivered via adeno-associated virus serotype 9.
References
- ↑ Le Prell, Colleen G. (3 August 2023). "Preclinical prospects of investigational agents for hearing loss treatment". Expert Opinion on Investigational Drugs. 32 (8): 685–692. doi:10.1080/13543784.2023.2253141. PMID 37695693. S2CID 261694986.
- ↑ Philippidis, Alex (1 December 2020). "Bayer, Novartis, Pfizer Raise Big Pharma's Stake in Gene Therapy". Human Gene Therapy. 31 (23–24): 1221–1223. doi:10.1089/hum.2020.29142.bfs. PMID 33337268. S2CID 229316419.
- ↑ Philippidis, Alex (1 January 2023). "StockWatch: Regeneron's All Ears for Hearing Loss Drug Developer: With planned up-to-$213M buyout, Decibel joins a growing number of biotechs focused on treating ear disorders to find a buyer". GEN Edge. 5 (1): 542–548. doi:10.1089/genedge.5.1.105. S2CID 265960410.
- ↑ Jiang, Luoying; Wang, Daqi; He, Yingzi; Shu, Yilai (April 2023). "Advances in gene therapy hold promise for treating hereditary hearing loss". Molecular Therapy. 31 (4): 934–950. doi: 10.1016/j.ymthe.2023.02.001 . PMC 10124073 . PMID 36755494.
- ↑ Development of an AAV-Based Gene Therapy for Children with Congenital Hearing Loss Due to Otoferlin Deficiency (DB-OTO). Source: Journal of Hearing Science . 2022, Vol. 12 Issue 1, p76-76. 1/4p. Author(s): De Raeve, L.; Holcomb, M.; Ramos Macías, A.
- ↑ Hayward, Eleanor (2024-05-09). "Toddler born deaf can hear after world-first gene therapy". The Times. ISSN 0140-0460 . Retrieved 2024-05-09.