Related Research Articles
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.

In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material.

Chain-growth polymerization (AE) or chain-growth polymerisation (BE) is a polymerization technique where monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
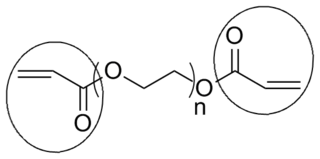
End groups are an important aspect of polymer synthesis and characterization. In polymer chemistry, they are functional groups that are at the very ends of a macromolecule or oligomer (IUPAC). In polymer synthesis, like condensation polymerization and free-radical types of polymerization, end-groups are commonly used and can be analyzed by nuclear magnetic resonance (NMR) to determine the average length of the polymer. Other methods for characterization of polymers where end-groups are used are mass spectrometry and vibrational spectrometry, like infrared and raman spectroscopy. These groups are important for the analysis of polymers and for grafting to and from a polymer chain to create a new copolymer. One example of an end group is in the polymer poly(ethylene glycol) diacrylate where the end-groups are circled.

In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.
In polymer chemistry, anionic addition polymerization is a form of chain-growth polymerization or addition polymerization that involves the polymerization of monomers initiated with anions. The type of reaction has many manifestations, but traditionally vinyl monomers are used. Often anionic polymerization involves living polymerizations, which allows control of structure and composition.

Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal catalyst. Polymerization from this method is called atom transfer radical addition polymerization (ATRAP). As the name implies, the atom transfer step is crucial in the reaction responsible for uniform polymer chain growth. ATRP was independently discovered by Mitsuo Sawamoto and by Krzysztof Matyjaszewski and Jin-Shan Wang in 1995.

Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization. It makes use of a chain-transfer agent (CTA) in the form of a thiocarbonylthio compound to afford control over the generated molecular weight and polydispersity during a free-radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) of Australia in 1998, RAFT polymerization is one of several living or controlled radical polymerization techniques, others being atom transfer radical polymerization (ATRP) and nitroxide-mediated polymerization (NMP), etc. RAFT polymerization uses thiocarbonylthio compounds, such as dithioesters, thiocarbamates, and xanthates, to mediate the polymerization via a reversible chain-transfer process. As with other controlled radical polymerization techniques, RAFT polymerizations can be performed under conditions that favor low dispersity and a pre-chosen molecular weight. RAFT polymerization can be used to design polymers of complex architectures, such as linear block copolymers, comb-like, star, brush polymers, dendrimers and cross-linked networks.
In polymer chemistry, chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.
In polymer chemistry, chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule:
In polymer chemistry, the kinetic chain length of a polymer is the average number of units called monomers added to a growing chain during chain-growth polymerization. During this process, a polymer chain is formed when monomers are bonded together to form long chains known as polymers. Kinetic chain length is defined as the average number of monomers that react with an active center such as a radical from initiation to termination.
Catalytic chain transfer (CCT) is a process that can be incorporated into radical polymerization to obtain greater control over the resulting products.
Cobalt based catalysts, when used in radical polymerization, have several main advantages especially in slowing down the reaction rate, allowing for the synthesis of polymers with peculiar properties. As starting the reaction does need a real radical initiator, the cobalt species is not the only used catalyst, it is a mediator. For this reason this type of reaction is referred to as cobalt mediated radical polymerization.

Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous.

Nitroxide-mediated radical polymerization is a method of radical polymerization that makes use of an nitroxide initiator to generate polymers with well controlled stereochemistry and a very low dispersity. It is a type of reversible-deactivation radical polymerization.

In polymer chemistry, reversible-deactivation radical polymerizations (RDRPs) are members of the class of reversible-deactivation polymerizations which exhibit much of the character of living polymerizations, but cannot be categorized as such as they are not without chain transfer or chain termination reactions. Several different names have been used in literature, which are:
In polymer chemistry, ionic polymerization is a chain-growth polymerization in which active centers are ions or ion pairs. It can be considered as an alternative to radical polymerization, and may refer to anionic polymerization or cationic polymerization.
An active center in polymer science refers to the site on a chain carrier at which reaction occurs.
In polymer chemistry, reversible-deactivation polymerization (RDP) is a form of polymerization propagated by chain carriers, some of which at any instant are held in a state of dormancy through an equilibrium process involving other species.
Copper-based reversible-deactivation radical polymerization is a member of the class of reversible-deactivation radical polymerization. In this system, various copper species are employed as the transition-metal catalyst for reversible activation/deactivation of the propagating chains responsible for uniform polymer chain growth.
References
- ↑ Aubrey, Jenkins; Jones, Richard G.; Moad, Graeme (2010). "Terminology for reversible-deactivation radical polymerization previously called "controlled" radical or "living" radical polymerization (IUPAC Recommendations 2010)". Pure Appl. Chem. 82 (2): 483–491. doi: 10.1351/PAC-REP-08-04-03 .
- ↑ Szwarc, M. (1956). "'Living' Polymers". Nature. 178 (4543): 1168–1169. doi:10.1038/1781168a0. ISSN 0028-0836.
- ↑ Szwarc, Michael (2000). "Comments on Living Polymerization: Rationale for Uniform Terminology? by Darling et al". Journal of Polymer Science Part A: Polymer Chemistry. 38 (10): 1710–1710. doi:10.1002/(SICI)1099-0518(20000515)38:10<1710::AID-POLA40>3.0.CO;2-K. ISSN 0887-624X.