A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a slightly different definition for biocides as "a diverse group of poisonous substances including preservatives, insecticides, disinfectants, and pesticides used for the control of organisms that are harmful to human or animal health or that cause damage to natural or manufactured products". When compared, the two definitions roughly imply the same, although the US EPA definition includes plant protection products and some veterinary medicines.

A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely used system for cataloguing information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product, along with spill-handling procedures. The older MSDS formats could vary from source to source within a country depending on national requirements; however, the newer SDS format is internationally standardized.

Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH.

The Toxic Substances Control Act is a United States law, passed by the 94th United States Congress in 1976 and administered by the United States Environmental Protection Agency (EPA), that regulates the introduction of new or already existing chemicals. When the TSCA was put into place, all existing chemicals were considered to be safe for use and subsequently grandfathered in. Its three main objectives are to assess and regulate new commercial chemicals before they enter the market, to regulate chemicals already existing in 1976 that posed an "unreasonable risk to health or to the environment", as for example PCBs, lead, mercury and radon, and to regulate these chemicals' distribution and use.

The European Chemicals Agency is an agency of the European Union which manages the technical and administrative aspects of the implementation of the European Union regulation called Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). ECHA is the driving force among regulatory authorities in implementing the EU's chemicals legislation. ECHA has to ascertain that companies comply with the legislation, advances the safe use of chemicals, provides information on chemicals and addresses chemicals of concern. It is located in Helsinki, Finland. ECHA is an independent and mature regulatory agency established by REACH. It is not a subsidiary entity of the European Commission.

The Control of Substances Hazardous to Health Regulations 2002 is a United Kingdom Statutory Instrument which states general requirements imposed on employers to protect employees and other persons from the hazards of substances used at work by risk assessment, control of exposure, health surveillance and incident planning. There are also duties on employees to take care of their own exposure to hazardous substances and prohibitions on the import of certain substances into the European Economic Area. The regulations reenacted, with amendments, the Control of Substances Hazardous to Work Regulations 1999 and implement several European Union directives.
High production volume chemicals are produced or imported into the United States in quantities of 1 million pounds or 500 tons per year. In OECD countries, HPV chemicals are defined as being produced at levels greater than 1,000 metric tons per producer/importer per year in at least one member country/region. A list of HPV chemicals serves as an overall priority list, from which chemicals are selected to gather data for a screening information dataset (SIDS), for testing and for initial hazard assessment.
An occupational exposure limit is an upper limit on the acceptable concentration of a hazardous substance in workplace air for a particular material or class of materials. It is typically set by competent national authorities and enforced by legislation to protect occupational safety and health. It is an important tool in risk assessment and in the management of activities involving handling of dangerous substances. There are many dangerous substances for which there are no formal occupational exposure limits. In these cases, hazard banding or control banding strategies can be used to ensure safe handling.
The Institute for Occupational Safety and Health of the German Social Accident Insurance is a German institute located in Sankt Augustin near Bonn and is a main department of the German Social Accident Insurance. Belonging to the Statutory Accident Insurance means that IFA is a non-profit institution.
The regulation of chemicals is the legislative intent of a variety of national laws or international initiatives such as agreements, strategies or conventions. These international initiatives define the policy of further regulations to be implemented locally as well as exposure or emission limits. Often, regulatory agencies oversee the enforcement of these laws.
Hazard statements form part of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). They are intended to form a set of standardized phrases about the hazards of chemical substances and mixtures that can be translated into different languages. As such, they serve the same purpose as the well-known R-phrases, which they are intended to replace.
A substance of very high concern (SVHC) is a chemical substance concerning which it has been proposed that use within the European Union be subject to authorisation under the REACH Regulation. Indeed, listing of a substance as an SVHC by the European Chemicals Agency (ECHA) is the first step in the procedure for authorisation or restriction of use of a chemical. The first list of SVHCs was published on 28 October 2008 and the list has been updated many times to include new candidates. The most recent update occurred in January 2022 to include a total of 223 SVHC.
A Chemical safety assessment (CSA) " is an analysis used in many situations where chemical are used and where there is a possibility that they may present a risk to life, health or the environment.
The Brandweerinformatiecentrum voor gevaarlijke stoffen/Information Centre for Dangerous Goods (BIG), established in the Flemish city of Geel, collects and validates information on dangerous goods.
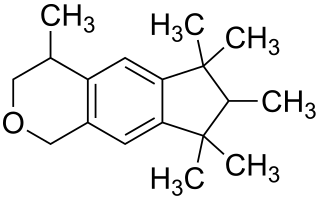
Galaxolide is a synthetic musk with a clean sweet musky floral woody odor used in fragrances. It is one of the musk components that perfume and cologne manufacturers use to add a musk odor to their products. Galaxolide was first synthesized in 1965, and used in the late 1960s in some fabric softeners and detergents. High concentrations were also incorporated in fine fragrances.
The authorisation procedure is one of the regulatory tools of the European regulation (EC) REACH n°1907/2006 aiming to ban the use of substances of very high concern (SVHC) included in the Annex XIV of REACH, so as to replace them with technically and economically feasible alternatives.
Occupational toxicology is the application of toxicology to chemical hazards in the workplace. It focuses on substances and conditions that occur in workplaces, where inhalation exposure and dermal exposure are most important, there is often exposure to mixtures of chemicals whose interactions are complex, health effects are influenced or confounded by other environmental and individual factors, and there is a focus on identifying early adverse affects that are more subtle than those presented in clinical medicine.

A pesticide, also called Plant Protection Product (PPP), which is a term used in regulatory documents, consists of several different components. The active ingredient in a pesticide is called “active substance” and these active substances either consist of chemicals or micro-organisms. The aims of these active substances are to specifically take action against organisms that are harmful to plants. In other words, active substances are the active components against pests and plant diseases.

The European Union Observatory for Nanomaterials (EUON) is an initiative that aims to increase the transparency and availability of information on nanomaterials to the general public. It was launched in June 2017.
Research on the health and safety hazards of 3D printing is new and in development due to the recent proliferation of 3D printing devices. In 2017, the European Agency for Safety and Health at Work has published a discussion paper on the processes and materials involved in 3D printing, potential implications of this technology for occupational safety and health and avenues for controlling potential hazards.







