
A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates the interior of a cell from the external environment or creates intracellular compartments by serving as a boundary between one part of the cell and another. Biological membranes, in the form of eukaryotic cell membranes, consist of a phospholipid bilayer with embedded, integral and peripheral proteins used in communication and transportation of chemicals and ions. The bulk of lipids in a cell membrane provides a fluid matrix for proteins to rotate and laterally diffuse for physiological functioning. Proteins are adapted to high membrane fluidity environment of the lipid bilayer with the presence of an annular lipid shell, consisting of lipid molecules bound tightly to the surface of integral membrane proteins. The cell membranes are different from the isolating tissues formed by layers of cells, such as mucous membranes, basement membranes, and serous membranes.

In cell biology, the cytoplasm describes all material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are the cytosol, the organelles, and various cytoplasmic inclusions. The cytoplasm is about 80% water and is usually colorless.

The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae, and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa.

The endomembrane system is composed of the different membranes (endomembranes) that are suspended in the cytoplasm within a eukaryotic cell. These membranes divide the cell into functional and structural compartments, or organelles. In eukaryotes the organelles of the endomembrane system include: the nuclear membrane, the endoplasmic reticulum, the Golgi apparatus, lysosomes, vesicles, endosomes, and plasma (cell) membrane among others. The system is defined more accurately as the set of membranes that forms a single functional and developmental unit, either being connected directly, or exchanging material through vesicle transport. Importantly, the endomembrane system does not include the membranes of plastids or mitochondria, but might have evolved partially from the actions of the latter.

The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.
A peroxisome (IPA:[pɛɜˈɹɒksɪˌsoʊm]) is a membrane-bound organelle, a type of microbody, found in the cytoplasm of virtually all eukaryotic cells. Peroxisomes are oxidative organelles. Frequently, molecular oxygen serves as a co-substrate, from which hydrogen peroxide (H2O2) is then formed. Peroxisomes owe their name to hydrogen peroxide generating and scavenging activities. They perform key roles in lipid metabolism and the conversion of reactive oxygen species. Peroxisomes are involved in the catabolism of very long chain fatty acids, branched chain fatty acids, bile acid intermediates (in the liver), D-amino acids, and polyamines, the reduction of reactive oxygen species – specifically hydrogen peroxide – and the biosynthesis of plasmalogens, i.e., ether phospholipids critical for the normal function of mammalian brains and lungs. They also contain approximately 10% of the total activity of two enzymes (Glucose-6-phosphate dehydrogenase and 6-Phosphogluconate dehydrogenase) in the pentose phosphate pathway, which is important for energy metabolism. It is vigorously debated whether peroxisomes are involved in isoprenoid and cholesterol synthesis in animals. Other known peroxisomal functions include the glyoxylate cycle in germinating seeds ("glyoxysomes"), photorespiration in leaves, glycolysis in trypanosomes ("glycosomes"), and methanol and/or amine oxidation and assimilation in some yeasts.
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs may consist of one or several alpha-helices or a transmembrane beta barrel. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in size and hydrophobicity; they may adopt organelle-specific properties.

Cytokinesis is the part of the cell division process during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or after the late stages of nuclear division in mitosis and meiosis. During cytokinesis the spindle apparatus partitions and transports duplicated chromatids into the cytoplasm of the separating daughter cells. It thereby ensures that chromosome number and complement are maintained from one generation to the next and that, except in special cases, the daughter cells will be functional copies of the parent cell. After the completion of the telophase and cytokinesis, each daughter cell enters the interphase of the cell cycle.
The translocon is a complex of proteins associated with the translocation of polypeptides across membranes. In eukaryotes the term translocon most commonly refers to the complex that transports nascent polypeptides with a targeting signal sequence into the interior space of the endoplasmic reticulum (ER) from the cytosol. This translocation process requires the protein to cross a hydrophobic lipid bilayer. The same complex is also used to integrate nascent proteins into the membrane itself. In prokaryotes, a similar protein complex transports polypeptides across the (inner) plasma membrane or integrates membrane proteins. In either case, the protein complex are formed from Sec proteins, with the heterotrimeric Sec61 being the channel. In prokaryotes, the homologous channel complex is known as SecYEG.

COPI is a coatomer, a protein complex that coats vesicles transporting proteins from the cis end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally synthesized, and between Golgi compartments. This type of transport is retrograde transport, in contrast to the anterograde transport associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the cis-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, δ, ε and ζ.
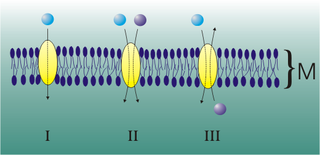
Mediated transport refers to transport mediated by a membrane transport protein. Substances in the human body may be hydrophobic, electrophilic, contain a positively or negatively charge, or have another property. As such there are times when those substances may not be able to pass over the cell membrane using protein-independent movement. The cell membrane is imbedded with many membrane transport proteins that allow such molecules to travel in and out of the cell. There are three types of mediated transporters: uniport, symport, and antiport. Things that can be transported are nutrients, ions, glucose, etc, all depending on the needs of the cell. One example of a uniport mediated transport protein is GLUT1. GLUT1 is a transmembrane protein, which means it spans the entire width of the cell membrane, connecting the extracellular and intracellular region. It is a uniport system because it specifically transports glucose in only one direction, down its concentration gradient across the cell membrane.

The sarcolemma also called the myolemma, is the cell membrane surrounding a skeletal muscle fiber or a cardiomyocyte. It consists of a lipid bilayer and a thin outer coat of polysaccharide material (glycocalyx) that contacts the basement membrane. The basement membrane contains numerous thin collagen fibrils and specialized proteins such as laminin that provide a scaffold to which the muscle fiber can adhere. Through transmembrane proteins in the plasma membrane, the actin skeleton inside the cell is connected to the basement membrane and the cell's exterior. At each end of the muscle fiber, the surface layer of the sarcolemma fuses with a tendon fiber, and the tendon fibers, in turn, collect into bundles to form the muscle tendons that adhere to bones.

Plasmodesmata are microscopic channels which traverse the cell walls of plant cells and some algal cells, enabling transport and communication between them. Plasmodesmata evolved independently in several lineages, and species that have these structures include members of the Charophyceae, Charales, Coleochaetales and Phaeophyceae, as well as all embryophytes, better known as land plants. Unlike animal cells, almost every plant cell is surrounded by a polysaccharide cell wall. Neighbouring plant cells are therefore separated by a pair of cell walls and the intervening middle lamella, forming an extracellular domain known as the apoplast. Although cell walls are permeable to small soluble proteins and other solutes, plasmodesmata enable direct, regulated, symplastic transport of substances between cells. There are two forms of plasmodesmata: primary plasmodesmata, which are formed during cell division, and secondary plasmodesmata, which can form between mature cells.
The following outline is provided as an overview of and topical guide to cell biology:
Reticulons are a group of evolutionary conservative proteins residing predominantly in endoplasmic reticulum, primarily playing a role in promoting membrane curvature. In addition, reticulons may play a role in nuclear pore complex formation, vesicle formation, and other processes yet to be defined. They have also been linked to oligodendrocyte roles in inhibition of neurite outgrowth. Some studies link RTNs with Alzheimer's disease and amyotrophic lateral sclerosis.
Membrane contact sites (MCS) are close appositions between two organelles. Ultrastructural studies typically reveal an intermembrane distance in the order of the size of a single protein, as small as 10 nm or wider, with no clear upper limit. These zones of apposition are highly conserved in evolution. These sites are thought to be important to facilitate signalling, and they promote the passage of small molecules, including ions, lipids and reactive oxygen species. MCS are important in the function of the endoplasmic reticulum (ER), since this is the major site of lipid synthesis within cells. The ER makes close contact with many organelles, including mitochondria, Golgi, endosomes, lysosomes, peroxisomes, chloroplasts and the plasma membrane. Both mitochondria and sorting endosomes undergo major rearrangements leading to fission where they contact the ER. Sites of close apposition can also form between most of these organelles most pairwise combinations. First mentions of these contact sites can be found in papers published in the late 1950s mainly visualized using electron microscopy (EM) techniques. Copeland and Dalton described them as “highly specialized tubular form of endoplasmic reticulum in association with the mitochondria and apparently in turn, with the vascular border of the cell”.
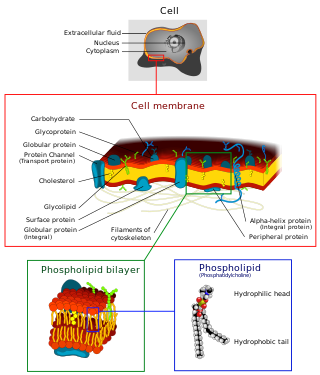
The cell membrane is a biological membrane that separates and protects the interior of a cell from the outside environment. The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of a cell, being selectively permeable to ions and organic molecules. In addition, cell membranes are involved in a variety of cellular processes such as cell adhesion, ion conductivity, and cell signalling and serve as the attachment surface for several extracellular structures, including the cell wall and the carbohydrate layer called the glycocalyx, as well as the intracellular network of protein fibers called the cytoskeleton. In the field of synthetic biology, cell membranes can be artificially reassembled.

Omegasome is a cell organelle consisting of lipid bilayer membranes enriched for phosphatidylinositol 3-phosphate, and related to a process of autophagy. It is a subdomain of the Endoplasmic Reticulum (ER), and has a morphology resembling the Greek capital letter Omega (Ω). Omegasomes are the sites from which phagophores form, which are sack-like structures that mature into autophagosomes, and fuse with lysosomes in order to degrade the contents of the autophagosomes. The formation of omegasomes depends on various factors, however in general, formation of omegasomes is increased as a response to starvation, and in some biochemical situations the presence of PI(3)P leads to the formation of omegasomes.
DP1/Yop1p is an integral membrane protein family that, along with the reticulons, is responsible for the shape of the tubular endoplasmic reticulum (ER) in yeast and mammalian cells. Furthermore, it is also believed that they might be involved in sheet ER formation.
KKXX and for some proteins XKXX is a target peptide motif located in the C terminus in the amino acid structure of a protein responsible for retrieval of endoplasmic reticulum (ER) membrane proteins to and from the Golgi apparatus. These ER membrane proteins are transmembrane proteins that are then embedded into the ER membrane after transport from the Golgi. This motif is exclusively cytoplasmic and interacts with the COPI protein complex to target the ER from the cis end of the Golgi apparatus by retrograde transport.













