
Diethanolamides are common ingredients used in cosmetics to act as a foaming agents or as emulsifiers. [1] Chemically, they are amides formed from diethanolamine and carboxylic acids, typically fatty acids.
Examples include:

Diethanolamides are common ingredients used in cosmetics to act as a foaming agents or as emulsifiers. [1] Chemically, they are amides formed from diethanolamine and carboxylic acids, typically fatty acids.
Examples include:

Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. Surfactants may function as emulsifiers, wetting agents, detergents, foaming agents, or dispersants. The word "surfactant" is a blend of surface-active agent, coined c. 1950.

Meringue is a type of dessert or candy, often associated with French, Italian, Swiss and Polish cuisines, traditionally made from whipped egg whites and sugar, and occasionally an acidic ingredient such as lemon, vinegar, or cream of tartar. A binding agent such as salt, flour or gelatin may also be added to the eggs. The key to the formation of a good meringue is the formation of stiff peaks by denaturing the protein ovalbumin via mechanical shear.

Angel food cake, or angel cake, is a type of sponge cake made with egg whites, flour, and sugar. A whipping agent, such as cream of tartar, is commonly added. It differs from other cakes because it uses no butter. Its aerated texture comes from whipped egg white. Angel food cake originated in the United States and first became popular in the late 19th century. It gained its unique reputation along with its name due to its light and fluffy texture.

Cocamide DEA, or cocamide diethanolamine, is a diethanolamide made by reacting the mixture of fatty acids from coconut oils with diethanolamine. It is a viscous liquid and is used as a foaming agent in bath products like shampoos and hand soaps, and in cosmetics as an emulsifying agent. See cocamide for the discussion of the lengths of carbon chains in the molecules in the mixture. The chemical formula of individual components is CH3(CH2)nC(=O)N(CH2CH2OH)2, where n typically ranges from 8 to 18.

Ethanolamine is an organic chemical compound with the formula HOCH
2CH
2NH
2 or C
2H
7NO. The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia. ETA molecules are a component in the formation of cellular membranes and are thus a molecular building block for life. It was thought to exist only on Earth and on certain asteroids, but in 2021 evidence was found that ETA molecules exist in interstellar space.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.

Piperazine is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.

Memory foam consists mainly of polyurethane with additional chemicals that increase its viscosity and density. It is often referred to as "viscoelastic" polyurethane foam, or low-resilience polyurethane foam (LRPu). The foam bubbles or ‘cells’ are open, effectively creating a matrix through which air can move. Higher-density memory foam softens in reaction to body heat, allowing it to mold to a warm body in a few minutes. Newer foams may recover their original shape more quickly.
Substances, mixtures and exposure circumstances in this list have been classified by the International Agency for Research on Cancer (IARC) as group 2B: The agent (mixture) is "possibly carcinogenic to humans". The exposure circumstance entails exposures that are possibly carcinogenic to humans. This category is used for agents, mixtures and exposure circumstances for which there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when there is inadequate evidence of carcinogenicity in humans but there is sufficient evidence of carcinogenicity in experimental animals. In some instances, an agent, mixture or exposure circumstance for which there is inadequate evidence of carcinogenicity in humans but limited evidence of carcinogenicity in experimental animals together with supporting evidence from other relevant data may be placed in this group. Further details can be found in the preamble to the IARC Monographs.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.

Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encountered as a colorless, viscous liquid. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans" (Group 2B).
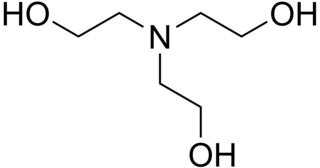
Triethanolamine, or TEA, is an organic compound with the chemical formula N(CH2CH2OH)3. It is a colourless viscous liquid. It is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999. It is a colourless compound although samples may appear yellow because of impurities.

Dichlorprop is a chlorophenoxy herbicide similar in structure to 2,4-D that is used to kill annual and perennial broadleaf weeds. It is a component of many common weedkillers. About 4 million pounds of dichlorprop are used annually in the United States.

A fire extinguisher is a handheld active fire protection device usually filled with a dry or wet chemical used to extinguish or control small fires, often in emergencies. It is not intended for use on an out-of-control fire, such as one which has reached the ceiling, endangers the user, or otherwise requires the equipment, personnel, resources, and/or expertise of a fire brigade. Typically, a fire extinguisher consists of a hand-held cylindrical pressure vessel containing an agent that can be discharged to extinguish a fire. Fire extinguishers manufactured with non-cylindrical pressure vessels also exist but are less common.

Methyl diethanolamine, also known as N-methyl diethanolamine and more commonly as MDEA, is the organic compound with the formula CH3N(C2H4OH)2. It is a colorless liquid with an ammonia odor. It is miscible with water, ethanol and benzene. A tertiary amine, it is widely used as a sweetening agent in chemical, oil refinery, syngas production and natural gas.
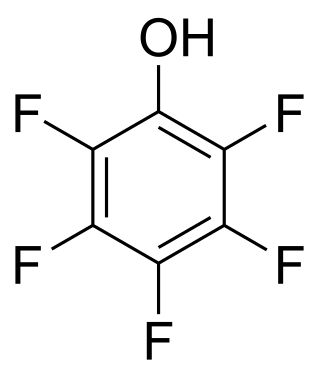
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound lacking C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method for their production. Due to their chemical stability, some of these perfluorinated compounds bioaccumulate.
MDEA may refer to:

Nifurquinazol (NF-1088) is an antibacterial agent of the nitrofuran class. It was never marketed.

G-130 is a drug with stimulant and anorectic effects, related to phenmetrazine.