The molecular mass (m) is the mass of a given molecule. The unit dalton (Da) is often used. Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The derived quantity relative molecular mass is the unitless ratio of the mass of a molecule to the atomic mass constant (which is equal to one dalton).

Size-exclusion chromatography, also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are commonly composed of dextran, agarose, or polyacrylamide polymers. The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.
Plate readers, also known as microplate readers or microplate photometers, are instruments which are used to detect biological, chemical or physical events of samples in microtiter plates. They are widely used in research, drug discovery, bioassay validation, quality control and manufacturing processes in the pharmaceutical and biotechnological industry and academic organizations. Sample reactions can be assayed in 1-1536 well format microtiter plates. The most common microplate format used in academic research laboratories or clinical diagnostic laboratories is 96-well with a typical reaction volume between 100 and 200 μL per well. Higher density microplates are typically used for screening applications, when throughput and assay cost per sample become critical parameters, with a typical assay volume between 5 and 50 μL per well. Common detection modes for microplate assays are absorbance, fluorescence intensity, luminescence, time-resolved fluorescence, and fluorescence polarization.

High-throughput screening (HTS) is a method for scientific discovery especially used in drug discovery and relevant to the fields of biology, materials science and chemistry. Using robotics, data processing/control software, liquid handling devices, and sensitive detectors, high-throughput screening allows a researcher to quickly conduct millions of chemical, genetic, or pharmacological tests. Through this process one can quickly recognize active compounds, antibodies, or genes that modulate a particular biomolecular pathway. The results of these experiments provide starting points for drug design and for understanding the noninteraction or role of a particular location.
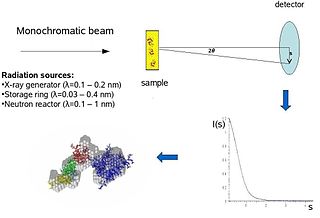
Biological small-angle scattering is a small-angle scattering method for structure analysis of biological materials. Small-angle scattering is used to study the structure of a variety of objects such as solutions of biological macromolecules, nanocomposites, alloys, and synthetic polymers. Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) are the two complementary techniques known jointly as small-angle scattering (SAS). SAS is an analogous method to X-ray and neutron diffraction, wide angle X-ray scattering, as well as to static light scattering. In contrast to other X-ray and neutron scattering methods, SAS yields information on the sizes and shapes of both crystalline and non-crystalline particles. When used to study biological materials, which are very often in aqueous solution, the scattering pattern is orientation averaged.

Surface plasmon resonance (SPR) is a phenomenon that occurs where electrons in a thin metal sheet become excited by light that is directed to the sheet with a particular angle of incidence, and then travel parallel to the sheet. Assuming a constant light source wavelength and that the metal sheet is thin, the angle of incidence that triggers SPR is related to the refractive index of the material and even a small change in the refractive index will cause SPR to not be observed. This makes SPR a possible technique for detecting particular substances (analytes) and SPR biosensors have been developed to detect various important biomarkers.
Nanoparticle tracking analysis (NTA) is a method for visualizing and analyzing particles in liquids that relates the rate of Brownian motion to particle size. The rate of movement is related only to the viscosity and temperature of the liquid; it is not influenced by particle density or refractive index. NTA allows the determination of a size distribution profile of small particles with a diameter of approximately 10–1000 nm in liquid suspension.
A particle counter is used for monitoring and diagnosing particle contamination within specific clean media, including air, water and chemicals. Particle counters are used in a variety of applications in support of clean manufacturing practices, industries include: electronic components and assemblies, pharmaceutical drug products and medical devices, and industrial technologies such as oil and gas.

Dynamic light scattering (DLS) is a technique in physics that can be used to determine the size distribution profile of small particles in suspension or polymers in solution. In the scope of DLS, temporal fluctuations are usually analyzed using the intensity or photon autocorrelation function. In the time domain analysis, the autocorrelation function (ACF) usually decays starting from zero delay time, and faster dynamics due to smaller particles lead to faster decorrelation of scattered intensity trace. It has been shown that the intensity ACF is the Fourier transform of the power spectrum, and therefore the DLS measurements can be equally well performed in the spectral domain. DLS can also be used to probe the behavior of complex fluids such as concentrated polymer solutions.
Hit to lead (H2L) also known as lead generation is a stage in early drug discovery where small molecule hits from a high throughput screen (HTS) are evaluated and undergo limited optimization to identify promising lead compounds. These lead compounds undergo more extensive optimization in a subsequent step of drug discovery called lead optimization (LO). The drug discovery process generally follows the following path that includes a hit to lead stage:
Static light scattering is a technique in physical chemistry that measures the intensity of the scattered light to obtain the average molecular weight Mw of a macromolecule like a polymer or a protein in solution. Measurement of the scattering intensity at many angles allows calculation of the root mean square radius, also called the radius of gyration Rg. By measuring the scattering intensity for many samples of various concentrations, the second virial coefficient, A2, can be calculated.
Particle size analysis, particle size measurement, or simply particle sizing, is the collective name of the technical procedures, or laboratory techniques which determines the size range, and/or the average, or mean size of the particles in a powder or liquid sample.
Multiangle light scattering (MALS) describes a technique for measuring the light scattered by a sample into a plurality of angles. It is used for determining both the absolute molar mass and the average size of molecules in solution, by detecting how they scatter light. A collimated beam from a laser source is most often used, in which case the technique can be referred to as multiangle laser light scattering (MALLS). The insertion of the word laser was intended to reassure those used to making light scattering measurements with conventional light sources, such as Hg-arc lamps that low-angle measurements could now be made. Until the advent of lasers and their associated fine beams of narrow width, the width of conventional light beams used to make such measurements prevented data collection at smaller scattering angles. In recent years, since all commercial light scattering instrumentation use laser sources, this need to mention the light source has been dropped and the term MALS is used throughout.
There are many methods to investigate protein–protein interactions which are the physical contacts of high specificity established between two or more protein molecules involving electrostatic forces and hydrophobic effects. Each of the approaches has its own strengths and weaknesses, especially with regard to the sensitivity and specificity of the method. A high sensitivity means that many of the interactions that occur are detected by the screen. A high specificity indicates that most of the interactions detected by the screen are occurring in reality.
Turbidimetry is the process of measuring the loss of intensity of transmitted light due to the scattering effect of particles suspended in it. Light is passed through a filter creating a light of known wavelength which is then passed through a cuvette containing a solution. A photoelectric cell collects the light which passes through the cuvette. A measurement is then given for the amount of absorbed light.
Richard Dale Smith is a chemist and a Battelle Fellow and chief scientist within the biological sciences division, as well as the director of proteomics research at the Pacific Northwest National Laboratory (PNNL). Smith is also director of the NIH Proteomics Research Resource for Integrative Biology, an adjunct faculty member in the chemistry departments at Washington State University and the University of Utah, and an affiliate faculty member at the University of Idaho and the Department of Molecular Microbiology & Immunology, Oregon Health & Science University. He is the author or co-author of approximately 1100 peer-reviewed publications and has been awarded 70 US patents.
A thermal shift assay (TSA) measures changes in the thermal denaturation temperature and hence stability of a protein under varying conditions such as variations in drug concentration, buffer pH or ionic strength, redox potential, or sequence mutation. The most common method for measuring protein thermal shifts is differential scanning fluorimetry (DSF) or thermofluor, which utilizes specialized fluorogenic dyes.
NanoSight Ltd is a company that designs and manufactures instruments for the scientific analysis of nanoparticles that are between approximately ten nanometers (nm) and one micron (μm) in diameter. The company was founded in 2003 by Bob Carr and John Knowles to further develop a technique Bob Carr had invented to visualize nanoparticles suspended in liquid. The company has since developed the technique of Nanoparticle Tracking Analysis (NTA), and they produce a series of instruments to count, size and visualize nanoparticles in liquid suspension using this patented technology.
Chemoproteomics entails a broad array of techniques used to identify and interrogate protein-small molecule interactions. Chemoproteomics complements phenotypic drug discovery, a paradigm that aims to discover lead compounds on the basis of alleviating a disease phenotype, as opposed to target-based drug discovery, in which lead compounds are designed to interact with predetermined disease-driving biological targets. As phenotypic drug discovery assays do not provide confirmation of a compound's mechanism of action, chemoproteomics provides valuable follow-up strategies to narrow down potential targets and eventually validate a molecule's mechanism of action. Chemoproteomics also attempts to address the inherent challenge of drug promiscuity in small molecule drug discovery by analyzing protein-small molecule interactions on a proteome-wide scale. A major goal of chemoproteomics is to characterize the interactome of drug candidates to gain insight into mechanisms of off-target toxicity and polypharmacology.
Microfluidic diffusional sizing (MDS) is a method to measure the size of particles based on the degree to which they diffuse within a microfluidic laminar flow. It allows size measurements to be taken from extremely small quantities of material (nano-grams) and is particularly useful when sizing molecules which may vary in size depending on their environment - e.g. protein molecules which may unfold or become denatured in unfavourable conditions.




