This page provides supplementary chemical data on diphenylamine.
This page provides supplementary chemical data on diphenylamine.
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity.

Dicofol is an organochlorine pesticide that is chemically related to DDT. Dicofol is a miticide that is very effective against spider mite. Its production and use is banned internationally under the Stockholm Convention.

Imidacloprid is a systemic insecticide belonging to a class of chemicals called the neonicotinoids which act on the central nervous system of insects. The chemical works by interfering with the transmission of stimuli in the insect nervous system. Specifically, it causes a blockage of the nicotinergic neuronal pathway. By blocking nicotinic acetylcholine receptors, imidacloprid prevents acetylcholine from transmitting impulses between nerves, resulting in the insect's paralysis and eventual death. It is effective on contact and via stomach action. Because imidacloprid binds much more strongly to insect neuron receptors than to mammal neuron receptors, this insecticide is more toxic to insects than to mammals.

Ethion (C9H22O4P2S4) is an organophosphate insecticide. Ethion is known to affect a neural enzyme called acetylcholinesterase and prevent it from working.

Toluene diisocyanate (TDI) is an organic compound with the formula CH3C6H3(NCO)2. Two of the six possible isomers are commercially important: 2,4-TDI (CAS: 584-84-9) and 2,6-TDI (CAS: 91-08-7). 2,4-TDI is produced in the pure state, but TDI is often marketed as 80/20 and 65/35 mixtures of the 2,4 and 2,6 isomers respectively. It is produced on a large scale, accounting for 34.1% of the global isocyanate market in 2000, second only to MDI. Approximately 1.4 billion kilograms were produced in 2000. All isomers of TDI are colorless, although commercial samples can appear yellow.

Heptachlor is an organochlorine compound that was used as an insecticide. Usually sold as a white or tan powder, heptachlor is one of the cyclodiene insecticides. In 1962, Rachel Carson's Silent Spring questioned the safety of heptachlor and other chlorinated insecticides. Due to its highly stable structure, heptachlor can persist in the environment for decades. In the United States, the Environmental Protection Agency has limited the sale of heptachlor products to the specific application of fire ant control in underground transformers. The amount that can be present in different foods is regulated.

Beryllium fluoride is the inorganic compound with the formula BeF2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.

D-Lysergic acid α-hydroxyethylamide, also known as D-lysergic acid methyl carbinolamide, is an alkaloid of the ergoline family, believed to be present in small amounts in various species in the Convolvulaceae, as well as some species of fungi.
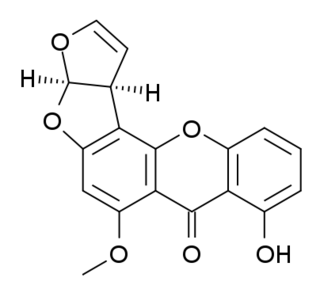
Sterigmatocystin is a polyketide mycotoxin produced by certain species of Aspergillus. The toxin is naturally found in some cheeses.

Hydramethylnon is an organofluorine compound. It is also known as AC 217,300. It is in a chemical class called trifluoromethyl aminohydrazone, which is a metabolic inhibitor. It is classified as a pesticide designed to control insects that are harmful to humans. It works by inhibiting complex III in the mitochondrial inner membrane and leads to a halting of oxidative phosphorylation. It is used primarily as an insecticide in the form of baits for cockroaches and ants. Some brands of insecticides that include hydramethylnon are Amdro, Blatex, Combat, Cyaforce, Cyclon, Faslane, Grant's, Impact, Matox, Maxforce, Pyramdron, Siege, Scuttle and Wipeout. Hydramethylnon is a slow-acting poison with delayed toxicity that needs to be eaten to be effective.
Poisonous material is a material, other than a gas, known to be so toxic to humans that it presents a health hazard during transportation.

Empenthrin (also called vaporthrin) is a synthetic pyrethroid used in insecticides. It is active against broad spectrum of flying insects including moths and other pests damaging textile. It has low acute mammalian toxicity (its oral LD50 is above 5000 mg/kg in male rats, above 3500 mg/kg in female rats and greater than 3500 mg/kg in mice). It is however very toxic to fish and other aquatic organisms (96-hour LC50 in Oncorhynchus mykiss is 1.7 μg/L, 48-hour EC50 in Daphnia magna is 20 μg/L).

Carbophenothion also known as Stauffer R 1303 as for the manufacturer, Stauffer Chemical, is an organophosphorus chemical compound. It was used as a pesticide for citrus fruits under the name of Trithion. Carbophenothion was used as an insecticide and acaricide. Although not used anymore it is still a restricted use pesticide in the United States. The chemical is identified in the US as an extremely hazardous substance according to the Emergency Planning and Community Right-to-Know Act.

Carbosulfan is an organic compound adherent to the carbamate class. At normal conditions, it is brown viscous liquid. It is not very stable; it decomposes slowly at room temperature. Its solubility in water is low but it is miscible with xylene, hexane, chloroform, dichloromethane, methanol and acetone. Carbosulfan is used as an insecticide. The European Union banned use of carbosulfan in 2007.

Tebufenpyrad is an insecticide and acaricide widely used in greenhouses. It is a white solid chemical with a slight aromatic smell. It is soluble in water and also in organic solvents.

EA-3990 is a deadly carbamate nerve agent. It is lethal because it inhibits acetylcholinesterase. Inhibition causes an overly high accumulation of acetylcholine between the nerve and muscle cells. This paralyzes the muscles by preventing their relaxation. The paralyzed muscles include the muscles used for breathing.

EA-4056 is a deadly carbamate nerve agent. It is lethal because it inhibits acetylcholinesterase. Inhibition causes an overly high accumulation of acetylcholine between the nerve and muscle cells. This paralyzes the muscles by preventing their relaxation. The paralyzed muscles includes the muscles used for breathing.
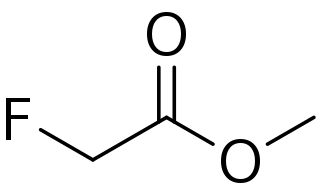
Methyl fluoroacetate (MFA) is an extremely toxic methyl ester of fluoroacetic acid. It is a colorless, odorless liquid at room temperature. It is used as a laboratory chemical and as a rodenticide. Because of its extreme toxicity, MFA was studied for potential use as a chemical weapon. The general population is not likely to be exposed to methyl fluoroacetate. People who use MFA for work, however, can breathe in or have direct skin contact with the substance.