Related Research Articles

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.

A lithium-ion battery or Li-ion battery is a type of rechargeable battery composed of cells in which lithium ions move from the negative electrode through an electrolyte to the positive electrode during discharge and back when charging. Li-ion cells use an intercalated lithium compound as the material at the positive electrode and typically graphite at the negative electrode. Li-ion batteries have a high energy density, no memory effect and low self-discharge. Cells can be manufactured to prioritize either energy or power density. They can however, be a safety hazard since they contain flammable electrolytes and, if damaged or incorrectly charged, can lead to explosions and fires.

A lithium polymer battery, or more correctly lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. High conductivity semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, such as mobile devices, radio-controlled aircraft and some electric vehicles.
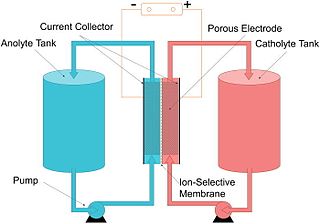
A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the electrodes.

Buckypaper is a thin sheet made from an aggregate of carbon nanotubes or carbon nanotube grid paper. The nanotubes are approximately 50,000 times thinner than a human hair. Originally, it was fabricated as a way to handle carbon nanotubes, but it is also being studied and developed into applications by several research groups, showing promise as vehicle armor, personal armor, and next-generation electronics and displays.

Lithium cobalt oxide, sometimes called lithium cobaltate or lithium cobaltite, is a chemical compound with formula LiCoO
2. The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide.

A lithium-ion capacitor (LIC) is a hybrid type of capacitor classified as a type of supercapacitor. It is called a hybrid because the anode is the same as those used in lithium-ion batteries and the cathode is the same as those used in supercapacitors. Activated carbon is typically used as the cathode. The anode of the LIC consists of carbon material which is often pre-doped with lithium ions. This pre-doping process lowers the potential of the anode and allows a relatively high output voltage compared to other supercapacitors.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.

A separator is a permeable membrane placed between a battery's anode and cathode. The main function of a separator is to keep the two electrodes apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current in an electrochemical cell.

Zinc–cerium batteries are a type of redox flow battery first developed by Plurion Inc. (UK) during the 2000s. In this rechargeable battery, both negative zinc and positive cerium electrolytes are circulated though an electrochemical flow reactor during the operation and stored in two separated reservoirs. Negative and positive electrolyte compartments in the electrochemical reactor are separated by a cation-exchange membrane, usually Nafion (DuPont). The Ce(III)/Ce(IV) and Zn(II)/Zn redox reactions take place at the positive and negative electrodes, respectively. Since zinc is electroplated during charge at the negative electrode this system is classified as a hybrid flow battery. Unlike in zinc–bromine and zinc–chlorine redox flow batteries, no condensation device is needed to dissolve halogen gases. The reagents used in the zinc-cerium system are considerably less expensive than those used in the vanadium flow battery.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.
Lithium hybrid organic batteries are an energy storage device that combines lithium with an organic polymer. For example, polyaniline vanadium (V) oxide (PAni/V2O5) can be incorporated into the nitroxide-polymer lithium iron phosphate battery, PTMA/LiFePO4. Together, they improve the lithium ion intercalation capacity, cycle life, electrochemical performances, and conductivity of batteries.

A solid dispersion redox flow battery is a type of redox flow battery using dispersed solid active materials as the energy storage media. The solid suspensions are stored in energy storage tanks and pumped through electrochemical cells while charging or discharging. In comparison with a conventional redox flow battery where active species are dissolved in aqueous or organic electrolyte, the active materials in a solid dispersion redox flow battery maintain the solid form and are suspended in the electrolyte. Further development expanded the applicable active materials. The solid active materials, especially with active materials from lithium-ion battery, can help the suspensions achieve much higher energy densities than conventional redox flow batteries. This concept is similar to semi-solid flow batteries in which slurries of active materials accompanied by conductive carbon additives to facilitate electrons conducting are stored in energy storage tanks and pumped through the electrochemical reaction cells. Based upon this technique, an analytical method was developed to measure the electrochemical performance of lithium-ion battery active materials, named dispersed particle resistance (DPR).

A semi-solid flow battery is a type of flow battery using solid battery active materials or involving solid species in the energy carrying fluid. A research team in MIT proposed this concept using lithium-ion battery materials. In such a system, both positive (cathode) and negative electrode (anode) consist of active material particles with carbon black suspended in liquid electrolyte. Active material suspensions are stored in two energy storage tanks. The suspensions are pumped into the electrochemical reaction cell when charging and discharging. This design takes advantage of both the designing flexibility of flow batteries and the high energy density active materials of lithium-ion batteries.

Doron Aurbach is an Israeli electrochemist, materials and surface scientist.
Scanning vibrating electrode technique (SVET), also known as vibrating probe within the field of biology, is a scanning probe microscopy (SPM) technique which visualizes electrochemical processes at a sample. It was originally introduced in 1974 by Jaffe and Nuccitelli to investigate the electrical current densities near living cells. Starting in the 1980s Hugh Isaacs began to apply SVET to a number of different corrosion studies. SVET measures local current density distributions in the solution above the sample of interest, to map electrochemical processes in situ as they occur. It utilizes a probe, vibrating perpendicular to the sample of interest, to enhance the measured signal. It is related to scanning ion-selective electrode technique (SIET), which can be used with SVET in corrosion studies, and scanning reference electrode technique (SRET), which is a precursor to SVET.

Kristina Edström is a Swedish Professor of Inorganic Chemistry at Uppsala University. She also serves as Head of the Ångström Advanced Battery Centre (ÅABC) and has previously been both Vice Dean for Research at the Faculty of Science and Technology and Chair of the STandUp for Energy research programme.
Calcium (ion) batteries are energy storage and delivery technologies (i.e., electro–chemical energy storage) that employ calcium ions (cations), Ca2+, as the active charge carrier in the electrolytes as well as in the electrodes (anode and cathode). Calcium (ion) batteries remain an active area of research, with studies and work persisting in the discovery and development of electrodes and electrolytes that enable stable, long-term battery operation.
Lithium nickel manganese cobalt oxides (abbreviated Li-NMC, LNMC, NMC or NCM) are mixed metal oxides of lithium, nickel, manganese and cobalt. They have the general formula LiNixMnyCozO2. The most important representatives have a composition with x + y + z that is near 1, with a small amount of lithium on the transition metal site. In commercial NMC samples, the composition typically has < 5% excess lithium. Structurally materials in this group are closely related to lithium cobalt(III) oxide (LiCoO2) and have a layered structure but possess an ideal charge distribution of Mn(IV), Co(III), and Ni(II) at the 1:1:1 stoichiometry. For more nickel-rich compositions, the nickel is in a more oxidized state for charge balance. NMCs are among the most important storage materials for lithium ions in lithium ion batteries. They are used on the positive side, which acts as the cathode during discharge.

A solid-state electrolyte (SSE) is a solid ionic conductor and electron-insulating material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery. The main advantages are the absolute safety, no issues of leakages of toxic organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte thanks to the property of lithium dendrite suppression in the presence of a solid-state electrolyte membrane. The utilization of a high capacity anode and low reduction potential, like lithium with a specific capacity of 3860 mAh g−1 and a reduction potential of -3.04 V vs SHE, in substitution of the traditional low capacity graphite, which exhibits a theoretical capacity of 372 mAh g−1 in its fully lithiated state of LiC6, is the first step in the realization of a lighter, thinner and cheaper rechargeable battery. Moreover this allows the reach of gravimetric and volumetric energy densities, high enough to achieve 500 miles per single charge in an electric vehicle. Despite the promising advantages, there are still many limitations that are hindering the transition of SSEs from academia research to large-scale production, depending mainly on the poor ionic conductivity compared to that of liquid counterparts. However, many car OEMs (Toyota, BMW, Honda, Hyundai) expect to integrate these systems into viable devices and to commercialize solid-state battery-based electric vehicles by 2025.
References
- ↑ Qi, Zhaoxiang; Koenig, Gary M. (2017-01-01). "Electrochemical Evaluation of Suspensions of Lithium-Ion Battery Active Materials as an Indicator of Rate Capability". Journal of the Electrochemical Society. 164 (2): A151–A155. doi:10.1149/2.0681702jes. ISSN 0013-4651. S2CID 99072168.
- ↑ Qi, Zhaoxiang; Dong, Hongxu; Koenig, Gary M. (2017-11-01). "Electrochemical Characterization of Lithium-Ion Battery Cathode Materials with Aqueous Flowing Dispersions". Electrochimica Acta. 253: 163–170. doi:10.1016/j.electacta.2017.09.031. ISSN 0013-4686.
- ↑ Geng, Linxiao; Denecke, Matthew E.; Foley, Sonia B.; Dong, Hongxu; Qi, Zhaoxiang; Koenig, Gary M. (2018-08-10). "Electrochemical characterization of lithium cobalt oxide within aqueous flow suspensions as an indicator of rate capability in lithium-ion battery electrodes". Electrochimica Acta. 281: 822–830. doi:10.1016/j.electacta.2018.06.037. ISSN 0013-4686. S2CID 103962594.