Related Research Articles

Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.
Dispersion may refer to:
In physics, attenuation is the gradual loss of flux intensity through a medium. For instance, dark glasses attenuate sunlight, lead attenuates X-rays, and water and air attenuate both light and sound at variable attenuation rates.
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.

Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The nanoparticles in the sintered material diffuse across the boundaries of the particles, fusing the particles together and creating a solid piece.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.
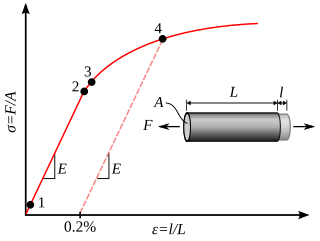
In physics and materials science, plasticity is the ability of a solid material to undergo permanent deformation, a non-reversible change of shape in response to applied forces. For example, a solid piece of metal being bent or pounded into a new shape displays plasticity as permanent changes occur within the material itself. In engineering, the transition from elastic behavior to plastic behavior is known as yielding.

A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.

Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material can strongly influence physical properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low temperature behaviour or wear resistance. These properties in turn govern the application of these materials in industrial practice.

Sir John Meurig Thomas, also known as JMT, was a Welsh scientist, educator, university administrator, and historian of science primarily known for his work on heterogeneous catalysis, solid-state chemistry, and surface and materials science.
A dispersion is a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter.

The Max-Planck-Institut für Kohlenforschung is an institute located in Mülheim an der Ruhr, Germany specializing in chemical research on catalysis. It is one of the 86 institutes in the Max Planck Society (Max-Planck-Gesellschaft). It was founded in 1912 in Mülheim an der Ruhr as the Kaiser Wilhelm Institute for Coal Research to study the chemistry and uses of coal, and became an independent Max Planck Institute in 1949.

Surface diffusion is a general process involving the motion of adatoms, molecules, and atomic clusters (adparticles) at solid material surfaces. The process can generally be thought of in terms of particles jumping between adjacent adsorption sites on a surface, as in figure 1. Just as in bulk diffusion, this motion is typically a thermally promoted process with rates increasing with increasing temperature. Many systems display diffusion behavior that deviates from the conventional model of nearest-neighbor jumps. Tunneling diffusion is a particularly interesting example of an unconventional mechanism wherein hydrogen has been shown to diffuse on clean metal surfaces via the quantum tunneling effect.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.

In chemistry, a catalyst support is a material, usually a solid with a high surface area, to which a catalyst is affixed. The activity of heterogeneous catalysts is mainly promoted by atoms present at the accessible surface of the material. Consequently, great effort is made to maximize the specific surface area of a catalyst. One popular method for increasing surface area involves distributing the catalyst over the surface of the support. The support may be inert or participate in the catalytic reactions. Typical supports include various kinds of activated carbon, alumina, and silica.
Zirconium phosphates (zirconium hydrogen phosphate) are acidic, inorganic cation exchange materials that have a layered structure with formula Zr(HPO4)2∙nH2O. These salts have high thermal and chemical stability, solid state ion conductivity, resistance to ionizing radiation, and the capacity to incorporate different types of molecules with different sizes between their layers. There are various phases of zirconium phosphate which vary in their interlaminar spaces and their crystalline structure. Among all the Zirconium phosphate phases the most widely used are the alpha (Zr(HPO4)2∙H2O) and the gamma (Zr(PO4)(H2PO4)∙2H2O) phase. The salts have been widely used in several applications such as: drug delivery, catalysis, nanocomposite, nuclear waste management, clinical dialyzer, among others.
The strength of metal oxide adhesion effectively determines the wetting of the metal-oxide interface. The strength of this adhesion is important, for instance, in production of light bulbs and fiber-matrix composites that depend on the optimization of wetting to create metal-ceramic interfaces. The strength of adhesion also determines the extent of dispersion on catalytically active metal. Metal oxide adhesion is important for applications such as complementary metal oxide semiconductor devices. These devices make possible the high packing densities of modern integrated circuits.

In heterogeneous catalysis, hydrogen molecules can be adsorbed and dissociated by the metal catalyst. Hydrogen spillover is the migration of hydrogen atoms from the metal catalyst onto the nonmetal support or adsorbate. Spillover, generally, is the transport of a species adsorbed or formed on a surface onto another surface. Hydrogen spillover can be characterized by three major steps, the first being where molecular hydrogen is split via dissociative chemisorption into its constitutive atoms on a transition metal catalyst surface, followed by migration from the catalyst to the substrate, culminating in their diffusion throughout the substrate surfaces and/or in the bulk materials.

Heterogeneous gold catalysis refers to the use of elemental gold as a heterogeneous catalyst. As in most heterogeneous catalysis, the metal is typically supported on metal oxide. Furthermore, as seen in other heterogeneous catalysts, activity increases with a decreasing diameter of supported gold clusters. Several industrially relevant processes are also observed such as H2 activation, Water-gas shift reaction, and hydrogenation. One or two gold-catalyzed reactions may have been commercialized.
References
- ↑ Bergeret, Gérard; Gallezot, Pierre (2008), "Particle Size and Dispersion Measurements" (PDF), Handbook of Heterogeneous Catalysis, American Chemical Society, pp. 738–765, doi:10.1002/9783527610044.hetcat0038, ISBN 978-3-527-61004-4