

Diterpene alkaloids are natural products of the terpene alkaloid type. [1]


Diterpene alkaloids are natural products of the terpene alkaloid type. [1]
Veatchine is found in the bark of Garrya veatchii , a member of the Cup Catkins family. [2] Aconitine is the main alkaloid in aconite. [3]
Diterpene alkaloids can be divided into two groups: The diterpene alkaloids, characterized by a C20 parent body, and the norditerpene alkaloids, which are based on a hexacyclic C19 parent body. [1]
Among the C20 alkaloids is the atisine-type (atisine, hetidine, hetisine) and the veatchine-type (Veatchin, Napellin). [1]
The C19 alkaloids include, among others, the aconitine type (aconitine, delphinine) and the lycoctonine type (Lycoctonin, Browniin). [1]
Aconitine has cardiac arrhythmic and antipyretic properties and is one of the most toxic plant compounds. [2]

Aconitine is an alkaloid toxin produced by various plant species belonging to the genus Aconitum, known also commonly by the names wolfsbane and monkshood. Monkshood is notorious for its toxic properties.

Toxiferine is a curare toxin. It is a bisindole alkaloid derived from Strychnos toxifera and a nicotinic acetylcholine receptor antagonist. This alkaloid is the main toxic component of Calabash curare, and one of the most toxic plant alkaloids known. The lethal dose (LD50) for mice has been determined as 10 - 60 µg/kg by intravenous administration. It is a muscle relaxant that causes paralysis of skeletal muscle, which takes approximately 2 hours to recovery for a moderate dose, and 8 hours of total paralysis with a 20-fold paralytic dose. The paralysis can be antagonized by neostigmine

Steroidal alkaloids have the basic steroidal skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic cyclopentanoperhydrophenanthrene skeleton that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in Chonemorpha fragrans, 'chonemorphine' was used to treat intestinal infections in Wistar rats..
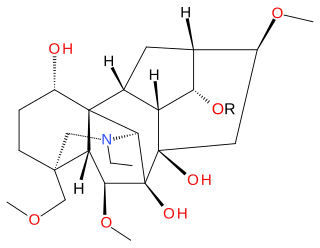
Delsoline and delcosine are two closely related naturally occurring diterpene alkaloids first isolated from Delphinium consolida. They occur widely in the Ranunculaceae plant family. The polycyclic ring system containing nineteen carbon atoms and one nitrogen atom in these compounds is the same as in aconitine and this is reflected in their preferred IUPAC name.

Karel František Wiesner was a Canadian chemist of Czech origin known for his contributions to the chemistry of natural products, notably aconitum alkaloids and digitalis glycosides.

Piperidine alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from piperidine.

Quinolizidine alkaloids are natural products that have a quinolizidine structure; this includes the lupine alkaloids.
Quinoline alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from quinoline. Some quinoline alkaloids show antiseptic, convulsive or antineoplastic effects.

The carbazole alkaloids are natural products of the indole alkaloid type, derived from carbazole.

Conium alkaloids are natural products of the piperidine alkaloid type.
The benzylisoquinoline alkaloids are natural products that can be classified as isoquinoline alkaloids and are derived from benzylisoquinoline. They also include the benzyl(tetrahydro)isoquinoline alkaloids.
Bisbenzylisoquinoline alkaloids are natural products found primarily in the plant families of the barberry family, the Menispermaceae, the Monimiaceae, and the buttercup family.

Cephalotaxus alkaloids are natural products characterized by pentacyclic structure.

Indolizidine alkaloids are natural products from various alkaloid groups whose structure can be derived from indolizidine.
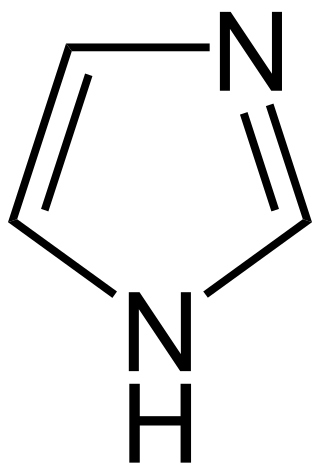
Imidazole alkaloids are a group of alkaloidss whose basic structure contains the imidazole ring system.

Corydalis Alkaloids are categorized as natural products of the isoquinoline alkaloid type.

Dendrobium alkaloids are natural products and so-called pseudoalkaloids.

The pyrrolidine alkaloids are natural products chemically derived from pyrrolidine.

Areca alkaloids are a group of piperidine alkaloids found in the areca nut, the seeds of the areca palm.
Apocynaceae alkaloids are natural products found in the plant family of the dogbane family (Apocynaceae).