Related Research Articles

Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat metastatic prostate cancer (mPC). To a lesser extent, it is used at high doses for locally advanced prostate cancer (LAPC) as a monotherapy without castration. Bicalutamide was also previously used as monotherapy to treat localized prostate cancer (LPC), but authorization for this use was withdrawn following unfavorable trial findings. Besides prostate cancer, bicalutamide is limitedly used in the treatment of excessive hair growth and scalp hair loss in women, as a puberty blocker and component of feminizing hormone therapy for transgender girls and women, to treat gonadotropin-independent early puberty in boys, and to prevent overly long-lasting erections in men. It is taken by mouth.
5α-Reductase inhibitors (5-ARIs), also known as dihydrotestosterone (DHT) blockers, are a class of medications with antiandrogenic effects which are used primarily in the treatment of enlarged prostate and scalp hair loss. They are also sometimes used to treat excess hair growth in women and as a component of hormone therapy for transgender women.

Dutasteride, sold under the brand name Avodart among others, is a medication primarily used to treat the symptoms of a benign prostatic hyperplasia (BPH), an enlarged prostate not associated with cancer. A few months may be required before benefits occur. It is also used for scalp hair loss in men and as a part of hormone therapy in transgender women. It is usually taken by mouth.

Flutamide, sold under the brand name Eulexin among others, is a nonsteroidal antiandrogen (NSAA) which is used primarily to treat prostate cancer. It is also used in the treatment of androgen-dependent conditions like acne, excessive hair growth, and high androgen levels in women. It is taken by mouth, usually three times per day.

Nilutamide, sold under the brand names Nilandron and Anandron, is a nonsteroidal antiandrogen (NSAA) which is used in the treatment of prostate cancer. It has also been studied as a component of feminizing hormone therapy for transgender women and to treat acne and seborrhea in women. It is taken by mouth.
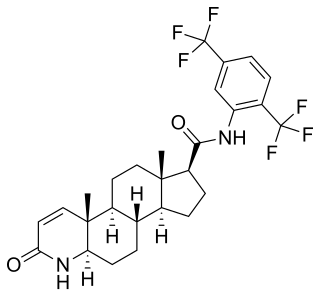
Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
Sipuleucel-T, sold under the brand name Provenge, developed by Dendreon Pharmaceuticals, LLC, is a cell-based cancer immunotherapy for prostate cancer (CaP). It is an autologous cellular immunotherapy.

Active surveillance is a management option for localized prostate cancer that can be offered to appropriate patients who would also be candidates for aggressive local therapies, with the intent to intervene if the disease progresses. Active surveillance should not be confused with watchful waiting, another observational strategy for men that would not be candidates for curative therapy because of a limited life expectancy. Active surveillance offers men with a prostate cancer that is thought to have a low risk of causing harm in the absence of treatment, a chance to delay or avoid aggressive treatment and its associated side effects.While prostate cancer is the most common non cutaneous cancer and second leading cause of cancer-related death in American men, it is conservatively estimated that approximately 100,000 men per year in the United States who would be eligible for conservative treatment through active surveillance, undergo unnecessary treatments. The management of localized prostate cancer is controversial and men with localized disease diagnosed today often undergo treatments with significant side effects that will not improve overall health outcomes. The 2011 NIH State-of-the-Science Conference Statement on the "Role of active surveillance in the management of men with localized prostate cancer" pointed out the many unanswered questions about observational strategies for prostate cancer that require further research and clarification. These included:
Sexual motivation is influenced by hormones such as testosterone, estrogen, progesterone, oxytocin, and vasopressin. In most mammalian species, sex hormones control the ability and motivation to engage in sexual behaviours.

Cyproterone acetate (CPA), sold alone under the brand name Androcur or with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent conditions such as acne, excessive body hair growth, early puberty, and prostate cancer, as a component of feminizing hormone therapy for transgender women, and in birth control pills. It is formulated and used both alone and in combination with an estrogen. CPA is taken by mouth one to three times per day.

A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically selective and full or silent antagonists of the androgen receptor (AR) and act by directly blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone.
Darolutamide, sold under the brand name Nubeqa, is an antiandrogen medication which is used in the treatment of non-metastatic castration-resistant prostate cancer in men. It is specifically approved to treat non-metastatic castration-resistant prostate cancer (nmCRPC) in conjunction with surgical or medical castration. The medication is taken by mouth twice per day with food.

Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.
The medical uses of bicalutamide, a nonsteroidal antiandrogen (NSAA), include the treatment of androgen-dependent conditions and hormone therapy to block the effects of androgens. Indications for bicalutamide include the treatment of prostate cancer in men, skin and hair conditions such as acne, seborrhea, hirsutism, and pattern hair loss in women, high testosterone levels in women, hormone therapy in transgender women, as a puberty blocker to prevent puberty in transgender girls and to treat early puberty in boys, and the treatment of long-lasting erections in men. It may also have some value in the treatment of paraphilias and hypersexuality in men.
The side effects of bicalutamide, a nonsteroidal antiandrogen (NSAA), including its frequent and rare side effects, have been well-studied and characterized. The most common side effects of bicalutamide monotherapy in men include breast tenderness, breast growth, feminization, demasculinization, and hot flashes. Less common side effects of bicalutamide monotherapy in men include sexual dysfunction, depression, fatigue, weakness, and anemia. Bicalutamide is well tolerated and has few side effects in women. General side effects of bicalutamide that may occur in either sex include diarrhea, constipation, abdominal pain, nausea, dry skin, itching, and rash.
Comparison of the nonsteroidal antiandrogen (NSAA) bicalutamide with other antiandrogens reveals differences between the medications in terms of efficacy, tolerability, safety, and other parameters. Relative to the other first-generation NSAAs, flutamide and nilutamide, bicalutamide shows improved potency, efficacy, tolerability, and safety, and has largely replaced these medications in clinical practice. Compared to the second-generation NSAAs, enzalutamide and apalutamide, bicalutamide has inferior potency and efficacy but similar tolerability and safety and a lower propensity for drug interactions.

The pharmacology of bicalutamide is the study of the pharmacodynamic and pharmacokinetic properties of the nonsteroidal antiandrogen (NSAA) bicalutamide. In terms of pharmacodynamics, bicalutamide acts as a selective antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has no capacity to activate the AR. It does not decrease androgen levels and has no other important hormonal activity. The medication has progonadotropic effects due to its AR antagonist activity and can increase androgen, estrogen, and neurosteroid production and levels. This results in a variety of differences of bicalutamide monotherapy compared to surgical and medical castration, such as indirect estrogenic effects and associated benefits like preservation of sexual function and drawbacks like gynecomastia. Bicalutamide can paradoxically stimulate late-stage prostate cancer due to accumulated mutations in the cancer. When used as a monotherapy, bicalutamide can induce breast development in males due to its estrogenic effects. Unlike other kinds of antiandrogens, it may have less adverse effect on the testes and fertility.
The Scandinavian Prostate Cancer Group (SPCG) is a group of scientific researchers who have conducted a series of clinical trials of treatments for prostate cancer. The group was founded in 1981 and the first study, SPCG-1, began in 1984.
The FinnProstate Group (FP), or FinnProstate Study Group, is a group of scientific researchers in Finland who have conducted a series of clinical trials of treatments for prostate cancer. The first publication by the group was in 1985 and the latest publication was in 2019.
References
- 1 2 3 4 Iversen P, Roder MA (March 2008). "The Early Prostate Cancer program: bicalutamide in nonmetastatic prostate cancer". Expert Rev Anticancer Ther. 8 (3): 361–9. doi:10.1586/14737140.8.3.361. PMID 18366284. S2CID 207189398.
- 1 2 3 4 5 6 7 8 9 Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer". Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. S2CID 46966712.
- 1 2 3 4 Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU Int. 91 (5): 455–61. doi: 10.1046/j.1464-410x.2003.04026.x . PMID 12603397.
- 1 2 3 4 See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, Chodak G, Montie J, Tyrrell C, Wallace DM, Delaere KP, Vaage S, Tammela TL, Lukkarinen O, Persson BE, Carroll K, Kolvenbag GJ (August 2002). "Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the early prostate cancer program". J Urol. 168 (2): 429–35. doi:10.1016/S0022-5347(05)64652-6. PMID 12131282.
- 1 2 Iversen P, McLeod DG, See WA, Morris T, Armstrong J, Wirth MP (April 2010). "Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide Early Prostate Cancer programme at a median follow-up of 9.7 years". BJU Int. 105 (8): 1074–81. doi:10.1111/j.1464-410X.2010.09319.x. PMID 22129214. S2CID 1261264.
- ↑ Fradet Y (February 2004). "Bicalutamide (Casodex) in the treatment of prostate cancer". Expert Rev Anticancer Ther. 4 (1): 37–48. doi:10.1586/14737140.4.1.37. PMID 14748655. S2CID 34153031.
- ↑ See WA, McLeod D, Iversen P, Wirth M (March 2001). "The bicalutamide Early Prostate Cancer Program. Demography". Urol Oncol. 6 (2): 43–47. doi:10.1016/s1078-1439(00)00118-6. PMID 11166619.
- ↑ McLeod DG, See WA, Klimberg I, Gleason D, Chodak G, Montie J, Bernstein G, Morris C, Armstrong J (July 2006). "The bicalutamide 150 mg early prostate cancer program: findings of the North American trial at 7.7-year median followup". J Urol. 176 (1): 75–80. doi:10.1016/S0022-5347(06)00495-2. PMID 16753373.
- ↑ Wirth M, Tyrrell C, Wallace M, Delaere KP, Sánchez-Chapado M, Ramon J, Hetherington J, Pina F, Heynes CF, Borchers TM, Morris T, Stone A (August 2001). "Bicalutamide (Casodex) 150 mg as immediate therapy in patients with localized or locally advanced prostate cancer significantly reduces the risk of disease progression". Urology. 58 (2): 146–51. doi:10.1016/s0090-4295(01)01213-4. PMID 11489683.
- ↑ Wirth M, Tyrrell C, Delaere K, Sánchez-Chapado M, Ramon J, Wallace DM, Hetherington J, Pina F, Heyns C, Borchers T, Morris T, Armstrong J (2005). "Bicalutamide ('Casodex') 150 mg in addition to standard care in patients with nonmetastatic prostate cancer: updated results from a randomised double-blind phase III study (median follow-up 5.1 y) in the early prostate cancer programme". Prostate Cancer Prostatic Dis. 8 (2): 194–200. doi: 10.1038/sj.pcan.4500799 . PMID 15931272.
- ↑ Wirth M, Tyrrell C, Delaere K, Sánchez-Chapado M, Ramon J, Wallace DM, Hetherington J, Pina F, Heyns CF, Navani S, Armstrong J (2007). "Bicalutamide (Casodex) 150 mg plus standard care in early non-metastatic prostate cancer: results from Early Prostate Cancer Trial 24 at a median 7 years' follow-up". Prostate Cancer Prostatic Dis. 10 (1): 87–93. doi: 10.1038/sj.pcan.4500916 . PMID 17102802.
- ↑ Iversen P, Tammela TL, Vaage S, Lukkarinen O, Lodding P, Bull-Njaa T, Viitanen J, Hoisaeter P, Lundmo P, Rasmussen F, Johansson JE, Persson BE, Carroll K (September 2002). "A randomised comparison of bicalutamide ('Casodex') 150 mg versus placebo as immediate therapy either alone or as adjuvant to standard care for early non-metastatic prostate cancer. First report from the Scandinavian Prostatic Cancer Group Study No. 6". Eur Urol. 42 (3): 204–11. doi:10.1016/s0302-2838(02)00311-1. PMID 12234503.
- 1 2 3 Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, Tammela TL, Tasdemir I, Morris T, Carroll K (November 2004). "Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6". J Urol. 172 (5 Pt 1): 1871–6. doi:10.1097/01.ju.0000139719.99825.54. PMID 15540741.
- ↑ Iversen P, Johansson JE, Lodding P, Kylmälä T, Lundmo P, Klarskov P, Tammela TL, Tasdemir I, Morris T, Armstrong J (2006). "Bicalutamide 150 mg in addition to standard care for patients with early non-metastatic prostate cancer: updated results from the Scandinavian Prostate Cancer Period Group-6 Study after a median follow-up period of 7.1 years". Scand J Urol Nephrol. 40 (6): 441–52. doi:10.1080/00365590601017329. PMID 17130095. S2CID 25862814.
- 1 2 Thomsen FB, Brasso K, Christensen IJ, Johansson JE, Angelsen A, Tammela TL, Iversen P (July 2015). "Survival benefit of early androgen receptor inhibitor therapy in locally advanced prostate cancer: long-term follow-up of the SPCG-6 study". Eur J Cancer. 51 (10): 1283–92. doi:10.1016/j.ejca.2015.03.021. PMID 25892647.
- ↑ Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K (November 2004). "Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years". J Urol. 172 (5 Pt 1): 1865–70. doi:10.1097/01.ju.0000140159.94703.80. PMID 15540740.
- ↑ McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP (February 2006). "Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer". BJU Int. 97 (2): 247–54. doi:10.1111/j.1464-410X.2005.06051.x. PMID 16430622.