Related Research Articles

Phosphoric acid, also known as orthophosphoric acid or phosphoric(V) acid, is a weak acid with the chemical formula H
3PO
4. It is normally encountered as a colorless syrup of 85% concentration in water. The pure compound is a colorless solid.
Polyphosphates are salts or esters of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic ring structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452.

The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones. Cyclic oximes and haloimines yield lactams.
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an industrial scale as a precursor to sulfuric acid.

Dinitrogen pentoxide is the chemical compound with the formula N2O5, also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 °C. Its boiling point is 47 °C, and sublimes slightly above room temperature, yielding a colorless gas.

Phosphorite,phosphate rock or rock phosphate is a non-detrital sedimentary rock that contains high amounts of phosphate minerals. The phosphate content of phosphorite (or grade of phosphate rock) varies greatly, from 4% to 20% phosphorus pentoxide (P2O5). Marketed phosphate rock is enriched ("beneficiated") to at least 28%, often more than 30% P2O5. This occurs through washing, screening, de-liming, magnetic separation or flotation. By comparison, the average phosphorus content of sedimentary rocks is less than 0.2%. The phosphate is present as fluorapatite Ca5(PO4)3F typically in cryptocrystalline masses (grain sizes < 1 μm) referred to as collophane-sedimentary apatite deposits of uncertain origin. It is also present as hydroxyapatite Ca5(PO4)3OH or Ca10(PO4)6(OH)2, which is often dissolved from vertebrate bones and teeth, whereas fluorapatite can originate from hydrothermal veins. Other sources also include chemically dissolved phosphate minerals from igneous and metamorphic rocks. Phosphorite deposits often occur in extensive layers, which cumulatively cover tens of thousands of square kilometres of the Earth's crust.

White phosphorus munitions are weapons which use one of the common allotropes of the chemical element phosphorus. White phosphorus is used in smoke, illumination, and incendiary munitions, and is commonly the burning element of tracer ammunition. Other common names include WP and the slang term "Willie Pete" or "Willie Peter" derived from William Peter, the World War II phonetic alphabet for "WP", which is still sometimes used in military jargon. White phosphorus is pyrophoric, burns fiercely, and can ignite cloth, fuel, ammunition, and other combustibles.

A phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus atom is in the oxidation state +5, and is bonded to four oxygen atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO
4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is H
n+2−2xP
nO
3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and (n+2)/2.

Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.

Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.

The Robinson–Gabriel synthesis is an organic reaction in which a 2-acylamino-ketone reacts intramolecularly followed by a dehydration to give an oxazole. A cyclodehydrating agent is needed to catalyze the reaction It is named after Sir Robert Robinson and Siegmund Gabriel who described the reaction in 1909 and 1910, respectively.

Phosphorus pentasulfide is the inorganic compound with the formula P2S5 or dimer P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF.
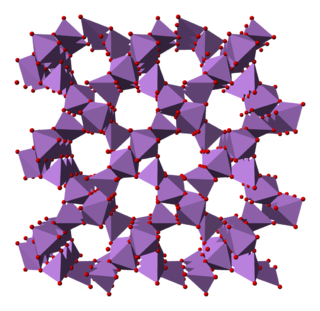
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All arsenic compounds are highly toxic and thus find only limited commercial applications.

Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.

Methanesulfonic acid (MsOH) or methanesulphonic acid (in British English) is a colorless liquid with the chemical formula CH3SO3H. It is the simplest of the alkylsulfonic acids. Salts and esters of methanesulfonic acid are known as mesylates (or methanesulfonates, as in ethyl methanesulfonate). It is hygroscopic in its concentrated form. Methanesulfonic acid may be considered an intermediate compound between sulfuric acid (H2SO4), and methylsulfonylmethane ((CH3)2SO2), effectively replacing an –OH group with a –CH3 group at each step. This pattern can extend no further in either direction without breaking down the –SO2– group. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher concentrations than in hydrochloric or sulfuric acid.

Methanesulfonic anhydride is the acid anhydride of methanesulfonic acid. Like methanesulfonyl chloride, it may be used to generate mesylates (methanesulfonyl esters). It is commercially available but may also be prepared by the reaction of phosphorus pentoxide with methanesulfonic acid at 80 °C.
The Thermphos International B.V. was a venture of the chemical industry, which produced phosphorus and inorganic phosphorus compounds. In 2005 it earned about 550 Million Euros and employed about 1200 people. It was Europe's only producer of elemental phosphorus. The company was also involved in recycling phosphorus. It used to be a former affiliate of the German chemicals company Hoechst AG and was taken over in 2003 by a group of private investors, led by the Italian-Israeli businessman Nahum Galmor.

Zinc–cerium batteries are a type of redox flow battery first developed by Plurion Inc. (UK) during the 2000s. In this rechargeable battery, both negative zinc and positive cerium electrolytes are circulated though an electrochemical flow reactor during the operation and stored in two separated reservoirs. Negative and positive electrolyte compartments in the electrochemical reactor are separated by a cation-exchange membrane, usually Nafion (DuPont). The Ce(III)/Ce(IV) and Zn(II)/Zn redox reactions take place at the positive and negative electrodes, respectively. Since zinc is electroplated during charge at the negative electrode this system is classified as a hybrid flow battery. Unlike in zinc–bromine and zinc–chlorine redox flow batteries, no condensation device is needed to dissolve halogen gases. The reagents used in the zinc-cerium system are considerably less expensive than those used in the vanadium flow battery.
Boron phosphate is an inorganic compound with the chemical formula BPO4. The simplest way of producing it is the reaction of phosphoric acid and boric acid. It is a white infusible solid that evaporates above 1450 °C.

Fluorophosphoric acid is the inorganic compound with the formula H2PO3F. It is a colorless viscous liquid that solidified to a glass upon cooling.
References
- ↑ Eaton, P. E.; Carlson, G. R.; Lee, J. T. (1973). "Phosphorus pentoxide-methanesulfonic acid. Convenient alternative to polyphosphoric acid". J. Org. Chem. 38 (23): 4071. doi:10.1021/jo00987a028.