
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), and is typically associated with the corrosion of refined iron.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".
The term chloride refers either to a chloride ion, which is negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond. Many inorganic chlorides are salts. Many organic compounds are chlorides. The pronunciation of the word "chloride" is.

Brine is a high-concentration solution of salt in water, containing 100,000 mg/L dissolved solids[. In diverse contexts, brine may refer to the salt solutions ranging from about 3.5% up to about 26%. Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride. Brine is used for food processing and cooking, for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization.
Passivation, in physical chemistry and engineering, refers to coating a material so it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. In electrochemical treatment of water, passivation reduces the effectiveness of the treatment by increasing the circuit resistance, and active measures are typically used to overcome this effect, the most common being polarity reversal, which results in limited rejection of the fouling layer.

Hot-dip galvanization is a form of galvanization. It is the process of coating iron and steel with zinc, which alloys with the surface of the base metal when immersing the metal in a bath of molten zinc at a temperature of around 450 °C (842 °F). When exposed to the atmosphere, the pure zinc (Zn) reacts with oxygen (O2) to form zinc oxide (ZnO), which further reacts with carbon dioxide (CO2) to form zinc carbonate (ZnCO3), a usually dull grey, fairly strong material that protects the steel underneath from further corrosion in many circumstances. Galvanized steel is widely used in applications where corrosion resistance is needed without the cost of stainless steel, and is considered superior in terms of cost and life-cycle. It can be identified by the crystallization patterning on the surface (often called a "spangle").

Pitting corrosion, or pitting, is a form of extremely localized corrosion that leads to the random creation of small holes in metal. The driving power for pitting corrosion is the depassivation of a small area, which becomes anodic while an unknown but potentially vast area becomes cathodic, leading to very localized galvanic corrosion. The corrosion penetrates the mass of the metal, with a limited diffusion of ions.
Tinplate consists of sheets of steel coated with a thin layer of tin to impede rusting. Before the advent of cheap milled steel, the backing metal was wrought iron. While once more widely used, the primary use of tinplate now is the manufacture of tin cans.

Tinning is the process of thinly coating sheets of wrought iron or steel with tin, and the resulting product is known as tinplate. The term is also widely used for the different process of coating a metal with solder before soldering.
Pickling is a metal surface treatment used to remove impurities, such as stains, inorganic contaminants, and rust or scale from ferrous metals, copper, precious metals and aluminum alloys. A solution called pickle liquor, which usually contains acid, is used to remove the surface impurities. It is commonly used to descale or clean steel in various steelmaking processes.
Bluing is a passivation process in which steel is partially protected against rust using a black oxide coating. It is named after the blue-black appearance of the resulting protective finish. Bluing involves an electrochemical conversion coating resulting from an oxidizing chemical reaction with iron on the surface selectively forming magnetite, the black oxide of iron. In comparison, rust, the red oxide of iron, undergoes an extremely large volume change upon hydration; as a result, the oxide easily flakes off, causing the typical reddish rusting away of iron. Black oxide provides minimal protection against corrosion, unless also treated with a water-displacing oil to reduce wetting and galvanic action. In colloquial use, thin coatings of black oxide are often termed 'gun bluing', while heavier coatings are termed 'black oxide'. Both refer to the same chemical process for providing true gun bluing.

Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).
Hydrochloric acid regeneration or HCl regeneration is a chemical process for the reclamation of bound and unbound HCl from metal chloride solutions such as hydrochloric acid.

Soda blasting is a mild form of abrasive blasting in which sodium bicarbonate particles are blasted against a surface using compressed air. It has a much milder abrasive effect than sandblasting. An early use was in the conservation-restoration of the Statue of Liberty in the 1980s.

Bronze disease is an irreversible and nearly inexorable corrosion process that occurs when chlorides come into contact with bronze or other copper-bearing alloys. It can occur as both a dark green coating, or as a much lighter whitish fuzzy or furry green coating. It is not a bacterial infection, but the result of a chemical reaction with the chlorides that usually occurs due to contamination of the bronze object by saltwater or from burial in specific types of soil where chloride salts are present. If not treated, complete destruction of the affected artifact is possible. Treatment is very difficult, costly and not always effective. Transfer of chlorides from the contaminated artefact to other artefacts can spread the condition.
Black powder is an industry name for the abrasive, reactive particulate contamination present in all gas and hydrocarbon fluid transmission lines. Black powder ranges from light brown to black, and the mineral makeup varies per production field around the world.

Smooth clean surface (SCS) is a process applied to hot rolled sheet metal and coils to remove nearly all mill scale and clean the steel surface.
Chlorine gas can be produced by extracting from natural materials, including the electrolysis of a sodium chloride solution (brine) and other ways.
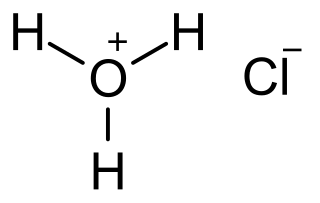
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical.













