Related Research Articles

Ammonia is a compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world's food and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.
A bactericide or bacteriocide, sometimes abbreviated Bcidal, is a substance which kills bacteria. Bactericides are disinfectants, antiseptics, or antibiotics. However, material surfaces can also have bactericidal properties based solely on their physical surface structure, as for example biomaterials like insect wings.

An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (—OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Trinitrotoluene, more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-Methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

Perchloric acid is a mineral acid with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.

Picric acid is an organic compound with the formula (O2N)3C6H2OH. Its IUPAC name is 2,4,6-trinitrophenol (TNP). The name "picric" comes from the Greek word πικρός (pikros), meaning "bitter", due to its bitter taste. It is one of the most acidic phenols. Like other strongly nitrated organic compounds, picric acid is an explosive, which is its primary use. It has also been used as medicine (antiseptic, burn treatments) and as a dye.

The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+
4. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic groups.

Ammonium nitrate is a chemical compound with the chemical formula NH4NO3. It is a white crystalline solid consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Global production was estimated at 21.6 million tonnes in 2017.
Cresols are a group of aromatic organic compounds. They are widely-occurring phenols which may be either natural or manufactured. They are also categorized as methylphenols. Cresols commonly occur as either solids or liquids because their melting points are generally close to room temperature. Like other types of phenols, they are slowly oxidized by exposure to air, and the resulting impurities often give the samples a yellow to brownish red tint. Cresols have an odor characteristic to that of other simple phenols, reminiscent to some of a "coal tar" smell. The name "cresol" is an adduct of phenol and their traditional source, creosote.

Amatol is a highly explosive material made from a mixture of TNT and ammonium nitrate. The British name originates from the words ammonium and toluene. Similar mixtures were known as Schneiderite in France. Amatol was used extensively during World War I and World War II, typically as an explosive in military weapons such as aircraft bombs, shells, depth charges, and naval mines. It was eventually replaced with alternative explosives such as Composition B, Torpex, and tritonal.

A shell is a projectile whose payload contains an explosive, incendiary, or other chemical filling. Originally it was called a bombshell, but "shell" has come to be unambiguous in a military context. Modern usage sometimes includes large solid kinetic projectiles that is properly termed shot. Solid shot may contain a pyrotechnic compound if a tracer or spotting charge is used.

Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid.
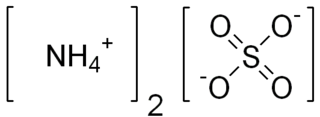
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed, usually as a base, in organic synthesis.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.

A picrate is a salt containing the anion (O2N)3C6H2O− or an ester derivative of the picrate anion. These salts are often produced by reactions of picric acid (2,4,6-trinitrophenol). The picrate ion is intensely yellow, although many of its salts are brown or orange-red.

Cleaning agents or hard-surface cleaners are substances used to remove dirt, including dust, stains, bad smells, and clutter on surfaces. Purposes of cleaning agents include health, beauty, removing offensive odor, and avoiding the spread of dirt and contaminants to oneself and others. Some cleaning agents can kill bacteria and clean at the same time. Others, called degreasers, contain organic solvents to help dissolve oils and fats.

A water-gel explosive is a fuel sensitized explosive mixture consisting of an aqueous ammonium nitrate solution that acts as the oxidizer. Water gels that are cap-insensitive are referred to under United States safety regulations as blasting agents. Water gel explosives have a jelly-like consistency and come in sausage-like packing stapled shut on both sides.
References
- ↑ Ann Vandermeer; Jeff VanderMeer (2 July 2019). The Big Book of Classic Fantasy. Knopf Doubleday Publishing Group. pp. 1310–. ISBN 978-0-525-43557-0.
- ↑ United States. Office of Naval Intelligence (1891). General Information Series ... U.S. Government Printing Office.
- ↑ Nitro-Explosives: A Practical Treatise by P. Gerald Sanford - Project Gutenberg