
Global warming potential (GWP) is an index to measure how much infrared thermal radiation a greenhouse gas would absorb over a given time frame after it has been added to the atmosphere. The GWP makes different greenhouse gases comparable with regard to their "effectiveness in causing radiative forcing". It is expressed as a multiple of the radiation that would be absorbed by the same mass of added carbon dioxide, which is taken as a reference gas. Therefore, the GWP has a value of 1 for CO2. For other gases it depends on how strongly the gas absorbs infrared thermal radiation, how quickly the gas leaves the atmosphere, and the time frame being considered.

Numerical climate models are mathematical models that can simulate the interactions of important drivers of climate. These drivers are the atmosphere, oceans, land surface and ice. Scientists use climate models to study the dynamics of the climate system and to make projections of future climate and of climate change. Climate models can also be qualitative models and contain narratives, largely descriptive, of possible futures.
Environmental science is an interdisciplinary academic field that integrates physics, biology, meteorology, mathematics and geography to the study of the environment, and the solution of environmental problems. Environmental science emerged from the fields of natural history and medicine during the Enlightenment. Today it provides an integrated, quantitative, and interdisciplinary approach to the study of environmental systems.

The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and stress corrosion cracking in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of H2O2 (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds.

Roger A. Pielke Sr. is an American meteorologist with interests in climate variability and climate change, environmental vulnerability, numerical modeling, atmospheric dynamics, land/ocean – atmosphere interactions, and large eddy/turbulent boundary layer modeling. He particularly focuses on mesoscale weather and climate processes but also investigates on the global, regional, and microscale.
Trace gases are gases that are present in small amounts within an environment such as a planet's atmosphere. Trace gases in Earth's atmosphere are gases other than nitrogen (78.1%), oxygen (20.9%), and argon (0.934%) which, in combination, make up 99.934% of its atmosphere.

Charles David Keeling was an American scientist whose recording of carbon dioxide at the Mauna Loa Observatory confirmed Svante Arrhenius's proposition (1896) of the possibility of anthropogenic contribution to the greenhouse effect and global warming, by documenting the steadily rising carbon dioxide levels. The Keeling Curve measures the progressive buildup of carbon dioxide, a greenhouse gas, in the atmosphere.

The Cooperative Institute for Research in Environmental Sciences (CIRES) is a research institute that is sponsored jointly by the National Oceanic and Atmospheric Administration (NOAA) Office of Oceanic and Atmospheric Research (OAR) and the University of Colorado Boulder (CU). CIRES scientists study the Earth system, including the atmosphere, hydrosphere, cryosphere, biosphere, and geosphere, and communicate these findings to decision makers, the scientific community, and the public.

The Max Planck Institute for Chemistry is a non-university research institute under the auspices of the Max Planck Society in Mainz, Germany. It was created as the Kaiser Wilhelm Institute for Chemistry in 1911 in Berlin.

Atmospheric dispersion modeling is the mathematical simulation of how air pollutants disperse in the ambient atmosphere. It is performed with computer programs that include algorithms to solve the mathematical equations that govern the pollutant dispersion. The dispersion models are used to estimate the downwind ambient concentration of air pollutants or toxins emitted from sources such as industrial plants, vehicular traffic or accidental chemical releases. They can also be used to predict future concentrations under specific scenarios. Therefore, they are the dominant type of model used in air quality policy making. They are most useful for pollutants that are dispersed over large distances and that may react in the atmosphere. For pollutants that have a very high spatio-temporal variability and for epidemiological studies statistical land-use regression models are also used.
Various governmental agencies involved with environmental protection and with occupational safety and health have promulgated regulations limiting the allowable concentrations of gaseous pollutants in the ambient air or in emissions to the ambient air. Such regulations involve a number of different expressions of concentration. Some express the concentrations as ppmv and some express the concentrations as mg/m3, while others require adjusting or correcting the concentrations to reference conditions of moisture content, oxygen content or carbon dioxide content. This article presents a set of useful conversions and formulas for air dispersion modeling of atmospheric pollutants and for complying with the various regulations as to how to express the concentrations obtained by such modeling.
The following outline is provided as an overview of and topical guide to air pollution dispersion: In environmental science, air pollution dispersion is the distribution of air pollution into the atmosphere. Air pollution is the introduction of particulates, biological molecules, or other harmful materials into Earth's atmosphere, causing disease, death to humans, damage to other living organisms such as food crops, and the natural or built environment. Air pollution may come from anthropogenic or natural sources. Dispersion refers to what happens to the pollution during and after its introduction; understanding this may help in identifying and controlling it.
In 2005, an international conference titled Avoiding Dangerous Climate Change: A Scientific Symposium on Stabilisation of Greenhouse Gases examined the link between atmospheric greenhouse gas concentration and global warming and its effects. The conference name was derived from Article 2 of the charter for the United Nations Framework Convention on Climate Change The conference explored the possible impacts at different levels of greenhouse gas emissions and how the climate might be stabilized at a desired level. The conference took place under the United Kingdom's presidency of the G8, with the participation of around 200 "internationally renowned" scientists from 30 countries. It was chaired by Dennis Tirpak and hosted by the Hadley Centre for Climate Prediction and Research in Exeter, from 1 February to 3 February. The conference was one of many meetings leading up to the 2015 Paris Agreement, at which the international community agreed to limit global warming to no more than 2 °C in order to have a 50-50 chance of avoiding dangerous climate change. However, a 2018 published study points at a threshold at which temperatures could rise to 4 or 5 degrees through self-reinforcing feedbacks in the climate system, suggesting the threshold is below the 2 degree temperature target.

The Mars general circulation model is the result of a research project by NASA to understand the nature of the general circulation of the atmosphere of Mars, how that circulation is driven and how it affects the climate of Mars in the long term.
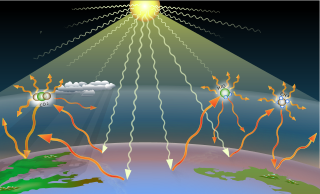
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F).
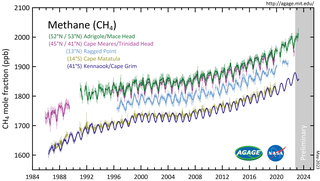
Atmospheric methane is the methane present in Earth's atmosphere. The concentration of atmospheric methane is increasing due to methane emissions, and is causing climate change. Methane is one of the most potent greenhouse gases. Methane's radiative forcing (RF) of climate is direct, and it is the second largest contributor to human-caused climate forcing in the historical period. Methane is a major source of water vapour in the stratosphere through oxidation; and water vapour adds about 15% to methane's radiative forcing effect. The global warming potential (GWP) for methane is about 84 in terms of its impact over a 20-year timeframe, and 28 in terms of its impact over a 100-year timeframe.

The history of the scientific discovery of climate change began in the early 19th century when ice ages and other natural changes in paleoclimate were first suspected and the natural greenhouse effect was first identified. In the late 19th century, scientists first argued that human emissions of greenhouse gases could change Earth's energy balance and climate. The existence of the greenhouse effect, while not named as such, was proposed as early as 1824 by Joseph Fourier. The argument and the evidence were further strengthened by Claude Pouillet in 1827 and 1838. In 1856 Eunice Newton Foote demonstrated that the warming effect of the sun is greater for air with water vapour than for dry air, and the effect is even greater with carbon dioxide.

Greenhouse gas monitoring is the direct measurement of greenhouse gas emissions and levels. There are several different methods of measuring carbon dioxide concentrations in the atmosphere, including infrared analyzing and manometry. Methane and nitrous oxide are measured by other instruments. Greenhouse gases are measured from space such as by the Orbiting Carbon Observatory and networks of ground stations such as the Integrated Carbon Observation System.

The atmospheric carbon cycle accounts for the exchange of gaseous carbon compounds, primarily carbon dioxide, between Earth's atmosphere, the oceans, and the terrestrial biosphere. It is one of the faster components of the planet's overall carbon cycle, supporting the exchange of more than 200 billion tons of carbon in and out of the atmosphere throughout the course of each year. Atmospheric concentrations of CO2 remain stable over longer timescales only when there exists a balance between these two flows. Methane, Carbon monoxide (CO), and other human-made compounds are present in smaller concentrations and are also part of the atmospheric carbon cycle.
Mace Head Atmospheric Research Station is located on the West Coast of Ireland in Carna, and is one of the longest running mercury recording stations in the world. The stations location is highly important as it is far away from neighbouring cities to ensure no pollutants interfere with recordings, and its location is also highly important as it is ideal for studying the atmosphere under Northern Hemispheric and European conditions. The station has the dual status of being a World Meteorological Organization (WMO) Global Atmosphere Watch (GMO) station and a European Monitoring and Evaluation Program (EMEP) supersite. Mace Head research and monitor the climate and atmospheric composition, focusing on aerosol-cloud interactions and mercury readings.














