Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's resistance to corrosion results from the chromium, which forms a passive film that can protect the material and self-heal in the presence of oxygen.

Cast iron is a class of iron–carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured; white cast iron has carbide impurities which allow cracks to pass straight through, grey cast iron has graphite flakes which deflect a passing crack and initiate countless new cracks as the material breaks, and ductile cast iron has spherical graphite "nodules" which stop the crack from further progressing.

A eutectic system or eutectic mixture is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the eutectic temperature. On a phase diagram, the eutectic temperature is seen as the eutectic point.

The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, which consumes only helium, and produces carbon. The alpha process most commonly occurs in massive stars and during supernovae.
In environmental chemistry, the chemical oxygen demand (COD) is an indicative measure of the amount of oxygen that can be consumed by reactions in a measured solution. It is commonly expressed in mass of oxygen consumed over volume of solution which in SI units is milligrams per litre (mg/L). A COD test can be used to easily quantify the amount of organics in water. The most common application of COD is in quantifying the amount of oxidizable pollutants found in surface water or wastewater. COD is useful in terms of water quality by providing a metric to determine the effect an effluent will have on the receiving body, much like biochemical oxygen demand (BOD).

Martensitic stainless steel is a type of stainless steel alloy that has a martensite crystal structure. It can be hardened and tempered through aging and heat treatment. The other main types of stainless steel are austenitic, ferritic, duplex, and precipitation hardened.

Maraging steels are steels that are known for possessing superior strength and toughness without losing ductility. Aging refers to the extended heat-treatment process. These steels are a special class of very-low-carbon ultra-high-strength steels that derive their strength not from carbon, but from precipitation of intermetallic compounds. The principal alloying element is 15 to 25 wt% nickel. Secondary alloying elements, which include cobalt, molybdenum and titanium, are added to produce intermetallic precipitates. Original development was carried out on 20 and 25 wt% Ni steels to which small additions of aluminium, titanium, and niobium were made; a rise in the price of cobalt in the late 1970s led to the development of cobalt-free maraging steels.

Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered at much higher temperatures.
Ferroalloy refers to various alloys of iron with a high proportion of one or more other elements such as manganese (Mn), aluminium (Al), or silicon (Si). They are used in the production of steels and alloys. The alloys impart distinctive qualities to steel and cast iron or serve important functions during production and are, therefore, closely associated with the iron and steel industry, the leading consumer of ferroalloys. The leading producers of ferroalloys in 2014 were China, South Africa, India, Russia and Kazakhstan, which accounted for 84% of the world production. World production of ferroalloys was estimated as 52.8 million tonnes in 2015.

In materials science, intergranular corrosion (IGC), also known as intergranular attack (IGA), is a form of corrosion where the boundaries of crystallites of the material are more susceptible to corrosion than their insides.
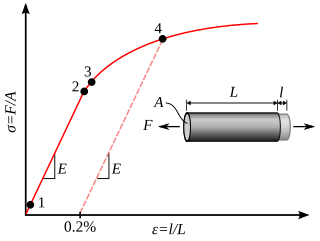
In materials science and engineering, the yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning of plastic behavior. Below the yield point, a material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible and is known as plastic deformation.
Resistance wire is wire intended for making electrical resistors. It is better if the alloy used has a high resistivity, since a shorter wire can then be used. In many situations, the stability of the resistor is of primary importance, and thus the alloy's temperature coefficient of resistivity and corrosion resistance play a large part in material selection.
Alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties.
In metalworking, a welding defect is any flaw that compromises the usefulness of a weldment. There is a great variety of welding defects. Welding imperfections are classified according to ISO 6520, while their acceptable limits are specified in ISO 5817 and ISO 10042.
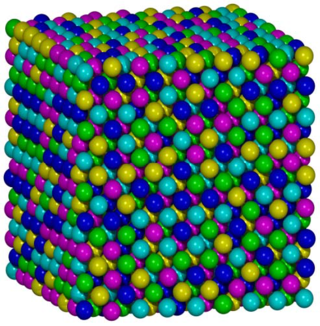
High-entropy alloys (HEAs) are alloys that are formed by mixing equal or relatively large proportions of (usually) five or more elements. Prior to the synthesis of these substances, typical metal alloys comprised one or two major components with smaller amounts of other elements. For example, additional elements can be added to iron to improve its properties, thereby creating an iron-based alloy, but typically in fairly low proportions, such as the proportions of carbon, manganese, and others in various steels. Hence, high-entropy alloys are a novel class of materials. The term "high-entropy alloys" was coined by Taiwanese scientist Jien-Wei Yeh because the entropy increase of mixing is substantially higher when there is a larger number of elements in the mix, and their proportions are more nearly equal. Some alternative names, such as multi-component alloys, compositionally complex alloys and multi-principal-element alloys are also suggested by other researchers.
Havar, or UNS R30004, is an alloy of cobalt, possessing a very high mechanical strength. It can be heat-treated. It is highly resistant to corrosion and is non-magnetic. It is biocompatible. It has high fatigue resistance. It is a precipitation hardening superalloy.
Friction hydro-pillar processing (FHPP) is a solid-state joining technology which can be used for filling of surface and sub-surface cracks in thick metals. For example, FHPP was attempted for the first time to repair cracks in space shuttle external components of high-strength aluminum alloys. FHPP is also considered in repairing surface cracks in steam turbine rotors of a high-strength, high-temperature-resistant steel (Ref). Alternative methods such as fusion welding processes for in-service repairing of cracks in components of these high-strength steels remained difficult because of their high hardenability and mandatory need of pre-heating and post-weld heat treatment. In contrast, initial FHPP trials could achieve joint strengths up to 90% of the base materials in high-strength steel components, especially those used for petrochemical and thermal power plants. In particular, pressurized pipes and vessels of AISI 4140 steel are widely used in the power generation, oil and gas, and petrochemical industries. Initial studies on FHPP of this alloy have showed promising results.





















