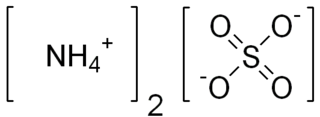Intermolecular forces (IMF) are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between molecules and other types of neighboring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics.

In chemistry, a salt is a solid chemical compound consisting of an ionic assembly of cations and anions. Salts are composed of related numbers of cations and anions so that the product is electrically neutral. These component ions can be inorganic, such as chloride (Cl−), or organic, such as acetate ; and can be monatomic, such as fluoride (F−) or polyatomic, such as sulfate.
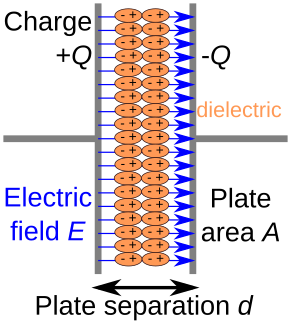
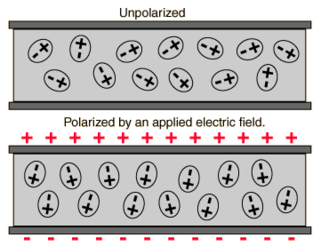
A dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric material is placed in an electric field, electric charges do not flow through the material as they do in an electrical conductor but only slightly shift from their average equilibrium positions causing dielectric polarization. Because of dielectric polarization, positive charges are displaced in the direction of the field and negative charges shift in the direction opposite to the field. This creates an internal electric field that reduces the overall field within the dielectric itself. If a dielectric is composed of weakly bonded molecules, those molecules not only become polarized, but also reorient so that their symmetry axes align to the field.
In electromagnetism, the absolute permittivity, often simply called permittivity and denoted by the Greek letter ε (epsilon), is a measure of the electric polarizability of a dielectric. A material with high permittivity polarizes more in response to an applied electric field than a material with low permittivity, thereby storing more energy in the electric field. In electrostatics, the permittivity plays an important role in determining the capacitance of a capacitor.
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation or with chemical reaction with another constituent of the solvent, such as acid or alkali. Each type of equilibrium is characterized by a temperature-dependent equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.

An acetate is a salt formed by the combination of acetic acid with an alkaline, earthy, metallic or nonmetallic and other base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.
Centrifugation is the technique which involves the application of centrifugal force to separate particles from a solution according to their size, shape, density, viscosity of the medium and rotor speed. Not only is this process used to separate two miscible substances, but also to analyze the hydrodynamic properties of macromolecules. More-dense components of the mixture migrate away from the axis of the centrifuge, while less-dense components of the mixture migrate towards the axis, i. e., move to the center. Chemists and biologists may increase the effective gravitational force on a test tube so as to more rapidly and completely cause the precipitate (pellet) to gather on the bottom of the tube. The remaining solution (supernatant) may be discarded with a pipette.. Centrifugation of protein solution, for example, allows elimination of impurities into the supernatant.

Precipitation is the creation of a solid from a solution. When the reaction occurs in a liquid solution, the solid formed is called the 'precipitate'. The chemical that causes the solid to form is called the 'precipitant'. Without sufficient force of gravity (settling) to bring the solid particles together, the precipitate remains in suspension. After sedimentation, especially when using a centrifuge to press it into a compact mass, the precipitate may be referred to as a 'pellet'. Precipitation can be used as a medium. The precipitate-free liquid remaining above the solid is called the 'supernate' or 'supernatant'. Powders derived from precipitation have also historically been known as 'flowers'. When the solid appears in the form of cellulose fibers which have been through chemical processing, the process is often referred to as regeneration.
The common-ion effect refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate. This behaviour is a consequence of Le Chatelier's principle for the equilibrium reaction of the ionic association/dissociation. The effect is commonly seen as an effect on the solubility of salts and other weak electrolytes. Adding an additional amount of one of the ions of the salt generally leads to increased precipitation of the salt, which reduces the concentration of both ions of the salt until the solubility equilibrium is reached. The effect is based on the fact that both the original salt and the other added chemical have one ion in common with each other.
DNA isolation is a process of purification of DNA from sample using a combination of physical and chemical methods. The first isolation of DNA was done in 1869 by Friedrich Miescher. Currently it is a routine procedure in molecular biology or forensic analyses. For the chemical method, there are many different kits used for extraction, and selecting the correct one will save time on kit optimization and extraction procedures. PCR sensitivity detection is considered to show the variation between the commercial kits.
Ammonium sulfate precipitation is one of the most commonly used methods for large and laboratory scale protein purification and fractionation that can be used to separate proteins by altering their solubility in the presence of a high salt concentration.
In plasmas and electrolytes, the Debye length, named after Peter Debye, is a measure of a charge carrier's net electrostatic effect in a solution and how far its electrostatic effect persists. A Debye sphere is a volume whose radius is the Debye length. With each Debye length, charges are increasingly electrically screened. Every Debye‐length , the electric potential will decrease in magnitude by 1/e. Debye length is an important parameter in plasma physics, electrolytes, and colloids. The corresponding Debye screening wave vector for particles of density , charge at a temperature is given by in Gaussian units. Expressions in MKS units will be given below. The analogous quantities at very low temperatures are known as the Thomas–Fermi length and the Thomas–Fermi wave vector. They are of interest in describing the behaviour of electrons in metals at room temperature.
In physics, the electric displacement field or electric induction is a vector field that appears in Maxwell's equations. It accounts for the effects of free and bound charge within materials. "D" stands for "displacement", as in the related concept of displacement current in dielectrics. In free space, the electric displacement field is equivalent to flux density, a concept that lends understanding to Gauss's law. In the International System of Units (SI), it is expressed in units of coulomb per meter square (C⋅m−2).
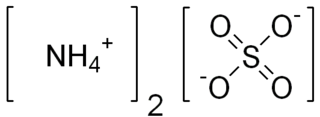
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

The chemists Peter Debye and Erich Hückel noticed that solutions that contain ionic solutes do not behave ideally even at very low concentrations. So, while the concentration of the solutes is fundamental to the calculation of the dynamics of a solution, they theorized that an extra factor that they termed gamma is necessary to the calculation of the activity coefficients of the solution. Hence they developed the Debye–Hückel equation and Debye–Hückel limiting law. The activity is only proportional to the concentration and is altered by a factor known as the activity coefficient . This factor takes into account the interaction energy of ions in solution.
Alkaline lysis or alkaline extraction is a method used in molecular biology to isolate plasmid DNA from bacteria.

Acid guanidinium thiocyanate-phenol-chloroform extraction is a liquid–liquid extraction technique in biochemistry. It is widely used in molecular biology for isolating RNA. This method may take longer than a column-based system such as the silica-based purification, but has higher purity and the advantage of high recovery of RNA: an RNA column is typically unsuitable for purification of short RNA species, such as siRNA, miRNA, gRNA and tRNA.
Protein precipitation is widely used in downstream processing of biological products in order to concentrate proteins and purify them from various contaminants. For example, in the biotechnology industry protein precipitation is used to eliminate contaminants commonly contained in blood. The underlying mechanism of precipitation is to alter the solvation potential of the solvent, more specifically, by lowering the solubility of the solute by addition of a reagent.
DNA separation by silica adsorption is a method of DNA separation that is based on DNA molecules binding to silica surfaces in the presence of certain salts and under certain pH conditions, usually conducted on a microchip coated in silica channels.
Chromatography is a physical method of separation that distributes the components you want to separate between two phases, one stationary, the other moving in a definite direction. Cold ethanol precipitation, developed by Cohn in 1946, manipulates pH, ionic strength, ethanol concentration and temperature to precipitate different protein fractions from plasma. Chromatographic techniques utilise ion exchange, gel filtration and affinity resins to separate proteins. Since the 1980s it has emerged as an effective method of purifying blood components for therapeutic use.