Related Research Articles

Biomedical engineering (BME) or medical engineering is the application of engineering principles and design concepts to medicine and biology for healthcare applications. BME is also traditionally logical sciences to advance health care treatment, including diagnosis, monitoring, and therapy. Also included under the scope of a biomedical engineer is the management of current medical equipment in hospitals while adhering to relevant industry standards. This involves procurement, routine testing, preventive maintenance, and making equipment recommendations, a role also known as a Biomedical Equipment Technician (BMET) or as a clinical engineer.

In medicine, a prosthesis, or a prosthetic implant, is an artificial device that replaces a missing body part, which may be lost through physical trauma, disease, or a condition present at birth. Prostheses are intended to restore the normal functions of the missing body part. Amputee rehabilitation is primarily coordinated by a physiatrist as part of an inter-disciplinary team consisting of physiatrists, prosthetists, nurses, physical therapists, and occupational therapists. Prostheses can be created by hand or with computer-aided design (CAD), a software interface that helps creators design and analyze the creation with computer-generated 2-D and 3-D graphics as well as analysis and optimization tools.
An artificial organ is a human made organ device or tissue that is implanted or integrated into a human — interfacing with living tissue — to replace a natural organ, to duplicate or augment a specific function or functions so the patient may return to a normal life as soon as possible. The replaced function does not have to be related to life support, but it often is. For example, replacement bones and joints, such as those found in hip replacements, could also be considered artificial organs.
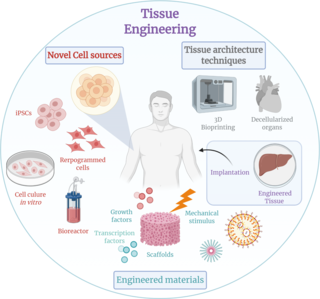
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can is considered as a field of its own.

Xenotransplantation, or heterologous transplant, is the transplantation of living cells, tissues or organs from one species to another. Such cells, tissues or organs are called xenografts or xenotransplants. It is contrasted with allotransplantation, syngeneic transplantation or isotransplantation and autotransplantation. Xenotransplantation is an artificial method of creating an animal-human chimera, that is, a human with a subset of animal cells. In contrast, an individual where each cell contains genetic material from a human and an animal is called a human–animal hybrid.

An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. For example, an implant may be a rod, used to strengthen weak bones. Medical implants are human-made devices, in contrast to a transplant, which is a transplanted biomedical tissue. The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone, or apatite depending on what is the most functional. In 2018, for example, American Elements developed a nickel alloy powder for 3D printing robust, long-lasting, and biocompatible medical implants. In some cases implants contain electronics, e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents.

Organ printing utilizes techniques similar to conventional 3D printing where a computer model is fed into a printer that lays down successive layers of plastics or wax until a 3D object is produced. In the case of organ printing, the material being used by the printer is a biocompatible plastic. The biocompatible plastic forms a scaffold that acts as the skeleton for the organ that is being printed. As the plastic is being laid down, it is also seeded with human cells from the patient's organ that is being printed for. After printing, the organ is transferred to an incubation chamber to give the cells time to grow. After a sufficient amount of time, the organ is implanted into the patient.
The Wake Forest Institute for Regenerative Medicine (WFIRM) is a research institute affiliated with Wake Forest School of Medicine and located in Winston-Salem, North Carolina, United States

A nose prosthesis is a craniofacial prosthesis for someone who no longer has their original nose. Nose prostheses are designed by anaplastologists who have their patients referred to them by ear, nose, and throat doctors and plastic surgeons.

The Methuselah Foundation is an American-based global non-profit organization based in Springfield, Virginia, with a declared mission to "make 90 the new 50 by 2030" by supporting tissue engineering and regenerative medicine therapies. The organization was originally incorporated by David Gobel in 2001 as the Performance Prize Society, a name inspired by the British governments Longitude Act, which offered monetary rewards for anyone who could devise a portable, practical solution for determining a ship's longitude.

United Therapeutics Corporation is an American publicly traded biotechnology company and public benefit corporation listed on the NASDAQ under the symbol UTHR. It develops novel, life-extending technologies for patients in the areas of lung disease and organ manufacturing. United Therapeutics is co-headquartered in Silver Spring, Maryland and Research Triangle Park, North Carolina, with additional facilities in Magog and Bromont, Quebec; Melbourne and Jacksonville, Florida; Blacksburg, Virginia; and Manchester, New Hampshire.
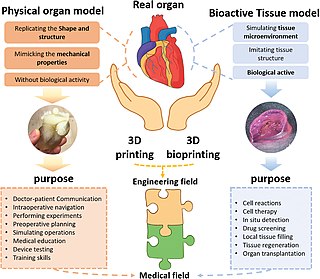
Three dimensional (3D) bioprinting is the utilization of 3D printing–like techniques to combine cells, growth factors, bio-inks, and biomaterials to fabricate functional structures that were traditionally used for tissue engineering applications but in recent times have seen increased interest in other applications such as biosensing, and environmental remediation. Generally, 3D bioprinting utilizes a layer-by-layer method to deposit materials known as bio-inks to create tissue-like structures that are later used in various medical and tissue engineering fields. 3D bioprinting covers a broad range of bioprinting techniques and biomaterials. Currently, bioprinting can be used to print tissue and organ models to help research drugs and potential treatments. Nonetheless, translation of bioprinted living cellular constructs into clinical application is met with several issues due to the complexity and cell number necessary to create functional organs. However, innovations span from bioprinting of extracellular matrix to mixing cells with hydrogels deposited layer by layer to produce the desired tissue. In addition, 3D bioprinting has begun to incorporate the printing of scaffolds which can be used to regenerate joints and ligaments. Apart from these, 3D bioprinting has recently been used in environmental remediation applications, including the fabrication of functional biofilms that host functional microorganisms that can facilitate pollutant removal.
In bioethics, ethics of organ transplantation refers to the ethical concerns on organ transplantation procedures. Both the source and method of obtaining the organ to transplant are major ethical issues to consider, as well as the notion of distributive justice.
Organovo Holdings, Inc. is an early-stage medical laboratory and research company which designs and develops functional, three dimensional human tissue for medical research and therapeutic applications. Organovo was established in 2007 and is headquartered in San Diego, California. The company uses its internally developed NovoGen MMX Bioprinter for 3D bioprinting.
Bio-inks are materials used to produce engineered/artificial live tissue using 3D printing. These inks are mostly composed of the cells that are being used, but are often used in tandem with additional materials that envelope the cells. The combination of cells and usually biopolymer gels are defined as a bio-ink. They must meet certain characteristics, including such as rheological, mechanical, biofunctional and biocompatibility properties, among others. Using bio-inks provides a high reproducibility and precise control over the fabricated constructs in an automated manner. These inks are considered as one of the most advanced tools for tissue engineering and regenerative medicine (TERM).

In recent years, 3D printing has developed significantly and can now perform crucial roles in many applications, with the most common applications being manufacturing, medicine, architecture, custom art and design, and can vary from fully functional to purely aesthetic applications.
Bico Group is a bioconvergence startup that designs and supplies technologies and services to enhance biology research. It focuses on commercializing technologies for life science research as well as bioprinting, and its products often combine capabilities in artificial intelligence, robotics, multiomics, and diagnostics.
Due to the many regulations in the industry, the design of medical devices presents significant challenges from both engineering and legal perspectives.

Microgravity bioprinting is the utilization of 3D bioprinting techniques under microgravity conditions to fabricate highly complex, functional tissue and organ structures. The zero gravity environment circumvents some of the current limitations of bioprinting on Earth including magnetic field disruption and biostructure retention during the printing process. Microgravity bioprinting is one of the initial steps to advancing in space exploration and colonization while furthering the possibilities of regenerative medicine.
Bioprinting drug delivery is a method for producing drug delivery vehicles. It uses three-dimensional printing of biomaterials via additive manufacturing. Such vehicles are biocompatible, tissue-specific hydrogels or implantable devices. 3D bioprinting prints cells and biological molecules to form tissues, organs, or biological materials in a scaffold-free manner that mimics living human tissue. The technique allows targeted disease treatments with scalable and complex geometry.
References
- 1 2 3 Dodds, Susan (11 February 2015). "3D printing raises ethical issues in medicine". ABC Science.
- ↑ Williams, Rhiannon (29 January 2014). "3D printing human tissue and organs to 'spark ethics debate'". The Telegraph.
- ↑ "What is 3D Printing? The definitive guide". 3D Hubs. Retrieved 2018-05-06.
- ↑ "What is Bioprinting?". www.innovateus.net. Retrieved 2018-05-06.
- ↑ "ExplainingTheFuture.com : Bioprinting". www.explainingthefuture.com. Retrieved 2018-05-06.
- ↑ Lim, Alane (May 2, 2018). "What is Bioprinting?". ThoughtCo. Retrieved September 17, 2019.
- ↑ University, Santa Clara. "What is Ethics? - Markkula Center for Applied Ethics". www.scu.edu. Retrieved 2018-05-06.
- ↑ "Limb Loss Statistics - Amputee Coalition". Amputee Coalition. Retrieved 2018-05-06.
- ↑ "Facts and Myths – American Transplant Foundation". www.americantransplantfoundation.org. Retrieved 2018-05-06.
- ↑ Kelly, Elizabeth. "FDA Regulation of 3D Printed Organs and Associated Ethical Challenges" (PDF). Archived from the original (PDF) on February 6, 2018.
- ↑ Vermeulen, Niki; Haddow, Gill; Seymour, Tirion; Faulkner-Jones, Alan; Shu, Wenmiao (2017-03-20). "3D bioprint me: a socioethical view of bioprinting human organs and tissues". Journal of Medical Ethics. 43 (9): medethics–2015–103347. doi:10.1136/medethics-2015-103347. ISSN 0306-6800. PMC 5827711 . PMID 28320774.