Related Research Articles

Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

Sodium hypochlorite, commonly known in a dilute solution as (chlorine) bleach, is an alkaline inorganic chemical compound with the formula NaOCl, consisting of a sodium cation and a hypochlorite anion. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.

Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually handled as an aqueous solution. It is commonly used as a bleach. More recent developments have extended its applications in food processing and as a disinfectant.
The Deacon process, invented by Henry Deacon, is a process used during the manufacture of alkalis by the Leblanc process. Hydrogen chloride gas was converted to chlorine gas, which was then used to manufacture a commercially valuable bleaching powder, and at the same time the emission of waste hydrochloric acid was curtailed. To some extent this technically sophisticated process superseded the earlier manganese dioxide process.
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds containing at least one covalently bonded atom of chlorine. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

Sodium chlorate is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper.

Tosoh Corporation is a global chemical and specialty materials company. The company was founded in 1935 in Yamaguchi Prefecture, as Toyo Soda Manufacturing Co., Ltd., and in 1987 changed its name to Tosoh Corporation. Today, its corporate headquarters are in Tokyo, Japan.

Olin Corporation is an American manufacturer of ammunition, chlorine, and sodium hydroxide. The company traces its roots to two companies, both founded in 1892: Franklin W. Olin's Equitable Powder Company and the Mathieson Alkali Works. Accidents at Olin chemical plants have exposed employees and nearby residents to health hazards.
The World Chlorine Council (WCC) is an international network of national and regional trade associations representing the chlorine and chlorinated products industries in more than 27 countries. Members include chloralkali process associations such as Euro Chlor, Japan Soda Industry Association, Alkali Manufacturers' Association of India, and RusChlor. Members from the product sector include five vinyl producer associations, and the Halogenated Solvents Industry Alliance.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove colour (whitening) from fabric or fiber or to disinfect after cleaning. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".
Bleaching of wood pulp is the chemical processing of wood pulp to lighten its color and whiten the pulp. The primary product of wood pulp is paper, for which whiteness is an important characteristic. These processes and chemistry are also applicable to the bleaching of non-wood pulps, such as those made from bamboo or kenaf.

Hexachlorobutadiene, (often abbreviated as "HCBD") Cl2C=C(Cl)C(Cl)=CCl2, is a colorless liquid at room temperature that has an odor similar to that of turpentine. It is a chlorinated aliphatic diene with niche applications but is most commonly used as a solvent for other chlorine-containing compounds. Structurally, it has a 1,3-butadiene core, but fully substituted with chlorine atoms.
Chlorine gas can be produced by extracting from natural materials, including the electrolysis of a sodium chloride solution (brine) and other ways.

The environmental effects of paper are significant, which has led to changes in industry and behaviour at both business and personal levels. With the use of modern technology such as the printing press and the highly mechanized harvesting of wood, disposable paper became a relatively cheap commodity, which led to a high level of consumption and waste. The rise in global environmental issues such as air and water pollution, climate change, overflowing landfills and clearcutting have all lead to increased government regulations. There is now a trend towards sustainability in the pulp and paper industry as it moves to reduce clear cutting, water use, greenhouse gas emissions, fossil fuel consumption and clean up its influence on local water supplies and air pollution.

Pentachlorobenzene (PeCB) is a chlorobenzene compound with the molecular formula C6HCl5 which is a chlorinated aromatic hydrocarbon. It consists of a benzene ring substituted with five chlorine atoms. PeCB was once used industrially for a variety of uses, but because of environmental concerns there are currently no large scale uses of PeCB. Pentachlorobenzene is a known persistent organic pollutant (POP) and banned globally by the Stockholm Convention on Persistent Organic Pollutants in 2009.

Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid.
Hooker Chemical Company was an American firm producing chloralkali products from 1903 to 1968. In 1922, bought the S. Wander & Sons Company to sell lye and chlorinated lime. The company became notorious in 1977, when residents near its chemical waste site, Love Canal, reported extraordinarily high incidences of leukemia, birth defects, and other injuries. Although Hooker had sold its old chemical waste dump site to the Niagara Falls School Board in 1953, the company was held responsible as a result of a lawsuit thereafter.
A mixed oxidant solution (MOS) is a type of disinfectant that has many uses including disinfecting, sterilizing, and eliminating pathogenic microorganisms in water. An MOS may have advantages such as a higher disinfecting power, stable residual chlorine in water, elimination of biofilm, and safety. The main components of an MOS are chlorine and its derivatives, which are produced by electrolysis of sodium chloride. It may also contain high amounts of hydroxy radicals, chlorine dioxide, dissolved ozone, hydrogen peroxide and oxygen from which the name "mixed oxidant" is derived.
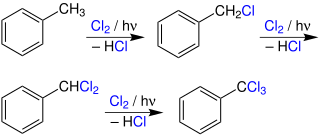
Photochlorination is a chlorination reaction that is initiated by light. Usually a C-H bond is converted to a C-Cl bond. Photochlorination is carried out on an industrial scale. The process is exothermic and proceeds as a chain reaction initiated by the homolytic cleavage of molecular chlorine into chlorine radicals by ultraviolet radiation. Many chlorinated solvents are produced in this way.
References
- 1 2 "About us". Euro Chlor. Archived from the original on 6 March 2020.
- 1 2 3 4 Amling, Andreas; Wehlage, Thomas (September 2014). "25 years" (PDF). Euro Chlor. Archived (PDF) from the original on 22 February 2020.
- 1 2 3 McMeekin, Andrew (2001). "6. Shaping the selection environment: 'chlorine in the dock'". In Coombs, Rod; Green, Ken; Richards, Albert; Walsh, Vivien (eds.). Technology and the Market: Demand, Users and Innovation. Edward Elgar Publishing. ISBN 1-84064-469-9.
- ↑ "About WCC". World Chlorine Council. Archived from the original on 6 March 2020.
- ↑ Amato, I. (9 July 1993). "The crusade against chlorine". Science. 261 (5118): 152–154. Bibcode:1993Sci...261..152A. doi:10.1126/science.8327884. ISSN 0036-8075. PMID 8327884.
- ↑ Hulpke, Herwig; Koch, Herbert A.; Nießner, Reinhard, eds. (2000). ECF (in German) (2nd ed.). Stuttgart: Georg Thieme Verlag. p. 237. ISBN 3-13-736502-3.
{{cite encyclopedia}}:|work=ignored (help) - ↑ Walkier, J. A. (1992). "Chlorine Safety". In Wellington, T. C. (ed.). Modern Chlor-Alkali Technology. Vol. 5. Society of Chemical Industry. pp. 233–234. doi:10.1007/978-94-011-2880-3_20. ISBN 978-1-85166-778-9.