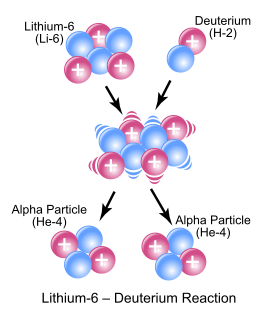Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles. The difference in mass between the reactants and products is manifested as either the release or the absorption of energy. This difference in mass arises due to the difference in nuclear binding energy between the atomic nuclei before and after the reaction. Nuclear fusion is the process that powers active or main sequence stars and other high-magnitude stars, where large amounts of energy are released.
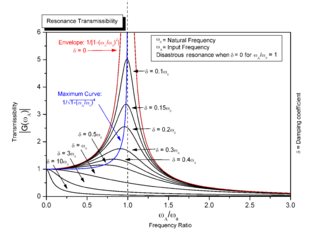
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force is equal or close to a natural frequency of the system on which it acts. When an oscillating force is applied at a resonant frequency of a dynamic system, the system will oscillate at a higher amplitude than when the same force is applied at other, non-resonant frequencies.

Photoluminescence is light emission from any form of matter after the absorption of photons. It is one of many forms of luminescence and is initiated by photoexcitation, hence the prefix photo-. Following excitation, various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductors up to milliseconds for phosphoresence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours.

Unbinilium, also known as eka-radium or simply element 120, is the hypothetical chemical element in the periodic table with symbol Ubn and atomic number 120. Unbinilium and Ubn are the temporary systematic IUPAC name and symbol, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be an s-block element, an alkaline earth metal, and the second element in the eighth period. It has attracted attention because of some predictions that it may be in the island of stability.

In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely more susceptible than fast neutrons to propagate a nuclear chain reaction of uranium-235 or other fissile isotope by colliding with their atomic nucleus.
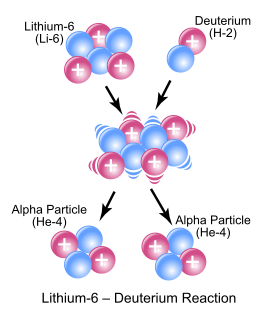
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. If a nucleus interacts with another nucleus or particle and they then separate without changing the nature of any nuclide, the process is simply referred to as a type of nuclear scattering, rather than a nuclear reaction.
Dynamic nuclear polarization (DNP) results from transferring spin polarization from electrons to nuclei, thereby aligning the nuclear spins to the extent that electron spins are aligned. Note that the alignment of electron spins at a given magnetic field and temperature is described by the Boltzmann distribution under the thermal equilibrium. It is also possible that those electrons are aligned to a higher degree of order by other preparations of electron spin order such as: chemical reactions, optical pumping and spin injection. DNP is considered one of several techniques for hyperpolarization. DNP can also be induced using unpaired electrons produced by radiation damage in solids.

Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.
A shape resonance is a metastable state in which an electron is trapped due to the shape of a potential barrier. Altunata describes a state as being a shape resonance if, "the internal state of the system remains unchanged upon disintegration of the quasi-bound level." A more general discussion of resonances and their taxonomies in molecular system can be found in the review article by Schulz,; for the discovery of the Fano resonance line-shape and for the Majorana pioneering work in this field by Antonio Bianconi; and for a mathematical review by Combes et al.
In physics, induced gamma emission (IGE) refers to the process of fluorescent emission of gamma rays from excited nuclei, usually involving a specific nuclear isomer. It is analogous to conventional fluorescence, which is defined as the emission of a photon by an excited electron in an atom or molecule. In the case of IGE, nuclear isomers can store significant amounts of excitation energy for times long enough for them to serve as nuclear fluorescent materials. There are over 800 known nuclear isomers but almost all are too intrinsically radioactive to be considered for applications. As of 2006 there were two proposed nuclear isomers that appeared to be physically capable of IGE fluorescence in safe arrangements: tantalum-180m and hafnium-178m2.
Dubnium (105Db) is a synthetic element, thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 261Db in 1968. The 13 known radioisotopes are from 255Db to 270Db, and 1–3 isomers. The longest-lived known isotope is 268Db with a half-life of 30 hours.
Seaborgium (106Sg) is a synthetic element and so has no stable isotopes. A standard atomic weight cannot be given. The first isotope to be synthesized was 263mSg in 1974. There are 12 known radioisotopes from 258Sg to 271Sg and 2 known isomers. The longest-lived isotope is 269Sg with a half-life of 14 minutes.
Meitnerium (109Mt) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 266Mt in 1982, and this is also the only isotope directly synthesized; all other isotopes are only known as decay products of heavier elements. There are eight known isotopes, from 266Mt to 278Mt. There may also be two isomers. The longest-lived of the known isotopes is 278Mt with a half-life of 8 seconds. The unconfirmed heavier 282Mt appears to have an even longer half-life of 67 seconds.
Copernicium (112Cn) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 277Cn in 1996. There are 6 known radioisotopes ; the longest-lived isotope is 285Cn with a half-life of 29 seconds.
Flerovium (114Fl) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 289Fl in 1999. Flerovium has seven known isotopes, and possibly 2 nuclear isomers. The longest-lived isotope is 289Fl with a half-life of 1.9 seconds, but the unconfirmed 290Fl may have a longer half-life of 19 seconds.
Moscovium (115Mc) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no known stable isotopes. The first isotope to be synthesized was 288Mc in 2004. There are five known radioisotopes from 286Mc to 290Mc. The longest-lived isotope is 290Mc with a half-life of 0.65 seconds.

Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer in 1958, consists of the nearly recoil-free emission and absorption of nuclear gamma rays in solids. The consequent nuclear spectroscopy method is exquisitely sensitive to small changes in the chemical environment of certain nuclei.
Giant resonance is a high-frequency collective excitation of atomic nuclei, as a property of many-body quantum systems. In the macroscopic interpretation of such an excitation in terms of an oscillation, the most prominent giant resonance is a collective oscillation of all protons against all neutrons in a nucleus.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).