Related Research Articles

The design of experiments is the design of any task that aims to describe or explain the variation of information under conditions that are hypothesized to reflect the variation. The term is generally associated with experiments in which the design introduces conditions that directly affect the variation, but may also refer to the design of quasi-experiments, in which natural conditions that influence the variation are selected for observation.
Epidemiology is the study and analysis of the distribution, patterns and determinants of health and disease conditions in defined populations.

A randomized controlled trial is a type of scientific experiment that aims to reduce certain sources of bias when testing the effectiveness of new treatments; this is accomplished by randomly allocating subjects to two or more groups, treating them differently, and then comparing them with respect to a measured response. One group—the experimental group—has the intervention being assessed, while the other—usually called the control group—has an alternative condition, such as a placebo or no intervention. The groups are followed under conditions of the trial design to see how effective the experimental intervention was. Treatment efficacy is assessed in comparison to the control. There may be more than one treatment group or more than one control group.
Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial – their approval does not mean that the therapy is 'safe' or effective, only that the trial may be conducted.
The science of epidemiology has matured significantly from the times of Hippocrates, Semmelweis and John Snow. The techniques for gathering and analyzing epidemiological data vary depending on the type of disease being monitored but each study will have overarching similarities.
A cohort study is a particular form of longitudinal study that samples a cohort, performing a cross-section at intervals through time. While a cohort study is a panel study, a panel study is not always a cohort study as individuals in a panel study do not always share a common characteristic.

A case–control study is a type of observational study in which two existing groups differing in outcome are identified and compared on the basis of some supposed causal attribute. Case–control studies are often used to identify factors that may contribute to a medical condition by comparing subjects who have that condition/disease with patients who do not have the condition/disease but are otherwise similar. They require fewer resources but provide less evidence for causal inference than a randomized controlled trial. We only get odds ratio from a case–control study, which is an inferior measure of strength of association as compared to relative risk.

The Women's Health Initiative (WHI) was initiated by the U.S. National Institutes of Health (NIH) in 1991. The Women's Health Initiative, which consisted of three clinical trials (CT) and an observational study (OS), was conducted to address major health issues causing morbidity and mortality in postmenopausal women. In particular, randomized controlled trials were designed and funded that addressed cardiovascular disease, cancer, and osteoporosis. In its entirety, the WHI enrolled more than 160,000 postmenopausal women aged 50–79 years over 15 years, making it one of the largest U.S. prevention studies of its kind, with a budget of $625 million. A 2014 analysis calculated a net economic return on investment of $37.1 billion for the estrogen-plus-progestin arm of the study's hormone trial alone, providing a strong case for the continued use of this variety of large, publicly funded population study.
In epidemiological research, recall bias is a systematic error caused by differences in the accuracy or completeness of the recollections retrieved ("recalled") by study participants regarding events or experiences from the past. Sometimes also referred to as response bias, responder bias or reporting bias, this type of measurement bias can be a methodological issue in research involving interviews or questionnaires, in which case it could lead to misclassification of various types of exposure. Recall bias is of particular concern in retrospective studies that use a case-control design to investigate the etiology of a disease or psychiatric condition. For example, in studies of risk factors for breast cancer, women who have had the disease may search their memories more thoroughly than members of the unaffected control group for possible causes of their cancer. Those in the case group may be able to recall a greater number of potential risk factors they had been exposed to than those in the control group. This can potentially exaggerate the relation between a potential risk factor and the disease. To minimize recall bias, some clinical trials have adopted a "wash out period", i.e., a substantial time period that must elapse between the subject's first observation and their subsequent observation of the same event.

A scientific control is an experiment or observation designed to minimize the effects of variables other than the independent variable. This increases the reliability of the results, often through a comparison between control measurements and the other measurements. Scientific controls are a part of the scientific method.
Clinical study design is the formulation of trials and experiments, as well as observational studies in medical, clinical and other types of research involving human beings. The goal of a clinical study is to assess the safety, efficacy, and / or the mechanism of action of an investigational medicinal product or procedure, or new drug or device that is in development, but potentially not yet approved by a health authority. It can also be to investigate a drug, device or procedure that has already been approved but is still in need of further investigation, typically with respect to long-term effects or cost-effectiveness.
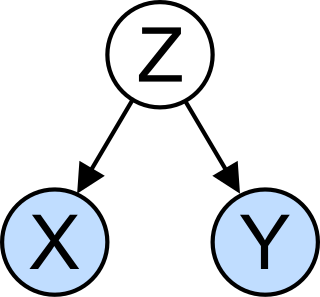
In statistics, a confounder is a variable that influences both the dependent variable and independent variable, causing a spurious association. Confounding is a causal concept, and as such, cannot be described in terms of correlations or associations.
In fields such as epidemiology, social sciences, psychology and statistics, an observational study draws inferences from a sample to a population where the independent variable is not under the control of the researcher because of ethical concerns or logistical constraints. One common observational study is about the possible effect of a treatment on subjects, where the assignment of subjects into a treated group versus a control group is outside the control of the investigator. This is in contrast with experiments, such as randomized controlled trials, where each subject is randomly assigned to a treated group or a control group.
The DASH diet is a dietary pattern promoted by the U.S.-based National Heart, Lung, and Blood Institute to prevent and control hypertension. The DASH diet is rich in fruits, vegetables, whole grains, and low-fat dairy foods; includes meat, fish, poultry, nuts, and beans; and is limited in sugar-sweetened foods and beverages, red meat, and added fats. In addition to its effect on blood pressure, it is designed to be a well-balanced approach to eating for the general public. DASH is recommended by the United States Department of Agriculture (USDA) as one of its ideal eating plans for all Americans.
In epidemiology, Mendelian randomization is a method of using measured variation in genes of known function to examine the causal effect of a modifiable exposure on disease in observational studies. The design was first proposed in 1986 and subsequently described by Gray and Wheatley as a method for obtaining unbiased estimates of the effects of a putative causal variable without conducting a traditional randomised trial. These authors also coined the term Mendelian randomization. The design has a powerful control for reverse causation and confounding, which often impede or mislead epidemiological studies.
A quasi-experiment is an empirical interventional study used to estimate the causal impact of an intervention on target population without random assignment. Quasi-experimental research shares similarities with the traditional experimental design or randomized controlled trial, but it specifically lacks the element of random assignment to treatment or control. Instead, quasi-experimental designs typically allow the researcher to control the assignment to the treatment condition, but using some criterion other than random assignment. In some cases, the researcher may have control over assignment to treatment. Quasi-experiments are subject to concerns regarding internal validity, because the treatment and control groups may not be comparable at baseline. With random assignment, study participants have the same chance of being assigned to the intervention group or the comparison group. As a result, differences between groups on both observed and unobserved characteristics would be due to chance, rather than to a systematic factor related to treatment. Randomization itself does not guarantee that groups will be equivalent at baseline. Any change in characteristics post-intervention is likely attributable to the intervention. With quasi-experimental studies, it may not be possible to convincingly demonstrate a causal link between the treatment condition and observed outcomes. This is particularly true if there are confounding variables that cannot be controlled or accounted for.
Pharmacoepidemiology is the study of the uses and effects of drugs in well-defined populations.
A glossary of terms used in clinical research.

The epidemiology of cancer is the study of the factors affecting cancer, as a way to infer possible trends and causes. The study of cancer epidemiology uses epidemiological methods to find the cause of cancer and to identify and develop improved treatments.
Prevention science is the application of a scientific methodology that seeks to prevent or moderate major human dysfunctions before they occur. Regardless of the type of issue on hand, the factors that lead to the problem must be identified and addressed. Prevention research is thus focused primarily on the systematic study of these potential precursors of dysfunction, also known as risk factors; as well as components or circumstances that reduces the probability of problem development in the presence of risk, also known as protective factors. Preventive interventions aim to counteract risk factors and reinforce protective factors in order to disrupt processes or situations that give rise to human or social dysfunction.
References
- 1 2 3 "Epidemiology and Public Health". courses.lumenlearning.com. Retrieved 23 March 2018.
- ↑ "experimental epidemiology". medical-dictionary.thefreedictionary.com. Retrieved 23 March 2018.
- ↑ Ahrens, Wolfgang; Pigeot, Iris (2007-07-26). Handbook of Epidemiology. Springer. p. 7. ISBN 9783540265771.
- ↑ Bonita, R.; Beaglehole, R.; Kjellström, Tord. "Basic Epidemiology" . Retrieved 23 March 2018.