
A dipeptide is an organic compound derived from two amino acids. The constituent amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Several dipeptides are physiologically important, and some are both physiologically and commercially significant. A well known dipeptide is aspartame, an artificial sweetener.

Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in alpha-amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents. Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.

Spirotryprostatin A is an indolic alkaloid from the 2,5-Diketopiperazine class of natural products found in the Aspergillus fumigatus fungus. Spirotryprostatin A and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
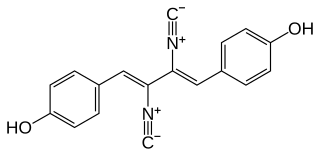
Xantocillin (INN), also known as xanthocillin X or ophthocillin, was the first reported natural product found to contain the isocyanide functional group. It was first isolated from Penicillium notatum by Rothe in 1950 and subsequently from several other sources.
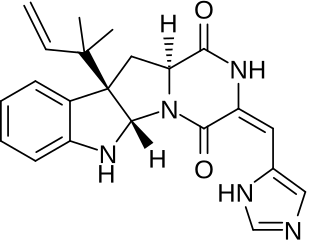
Roquefortine C is a mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by various fungi, particularly species from the genus Penicillium. It was first isolated from a strain of Penicillium roqueforti, a species commercially used as a source of proteolytic and lipolytic enzymes during maturation of the blue-veined cheeses, Roquefort, Danish Blue, Stilton and Gorgonzola.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.
2,5-Diketopiperazine is an organic compound with the formula (NHCH2C(O))2. The compound features a six-membered ring containing two amide groups at opposite positions in the ring. It was first compound containing a peptide bond to be characterized by X-ray crystallography in 1938. It is the parent of a large class of 2,5-Diketopiperazines (2,5-DKPs) with the formula (NHCH2(R)C(O))2 (R = H, CH3, etc.). They are ubiquitous peptide in nature. They are often found in fermentation broths and yeast cultures as well as embedded in larger more complex architectures in a variety of natural products as well as several drugs. In addition, they are often produced as degradation products of polypeptides, especially in processed foods and beverages. They have also been identified in the contents of comets.

(−)-Aurantiamine is a blue fluorescence metabolite produced by the fungus Penicillium aurantiogriseum, the most common fungi found in cereals. (−)-Aurantiamine belongs to a class of naturally occurring 2,5-diketopiperazines featuring a dehydrohistidine residue that exhibit important biological activities, such as anti-cancer or neurotoxic effects. It is the isopropyl analog of the microtubule binding agent (−)-phenylahistin but is 40 times less active than the latter on P388 cell proliferation. The total asymmetric synthesis of (−)-aurantiamine has been described.

Fumitremorgins are tremorogenic metabolites of Aspergillus and Penicillium, that belong to a class of naturally occurring 2,5-diketopiperazines.

Verruculogen is a mycotoxin produced by certain strains of aspergillus that belongs to a class of naturally occurring 2,5-diketopiperazines. It is an annulated analogue of cyclo(L-Trp-L-Pro) which belongs to the most abundant and structurally diverse class of tryptophan-proline 2,5-diketopiperazine natural products. It produces tremors in mice due to its neurotoxic properties. It also tested positive in a Salmonella/mammalian microsome assay and was shown to be genotoxic. It is a potent blocker of calcium-activated potassium channels.
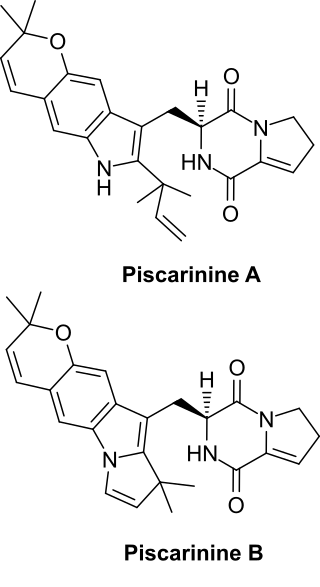
Piscarinines are bioactive alkaloid isolates of Penicillium piscarium NKM F-961 and Penicillium piscarium Westling that belong to a class of naturally occurring 2,5-diketopiperazines. The cytotoxic dehydroproline tryptophan derivatives piscarinines A and B were shown to be active against the prostate cancer cell line LNCAP.

Epoxyagroclavine is an ergot alkaloid made by permafrost Penicillium.
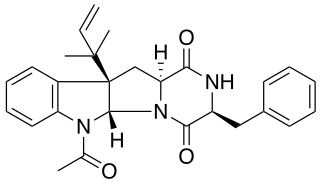
Rugulosuvines are bio-active alkaloids made by Penicillium, that belongs to a class of naturally occurring 2,5-diketopiperazines.

Stephacidin A and B are antitumor alkaloids isolated from the fungus Aspergillus ochraceus that belong to a class of naturally occurring 2,5-diketopiperazines. This unusual family of fungal metabolites are complex bridged 2,5-diketopiperazine alkaloids that possess a unique bicyclo[2.2.2]diazaoctane core ring system and are constituted mainly from tryptophan, proline, and substituted proline derivatives where the olefinic unit of the isoprene moiety has been formally oxidatively cyclized across the α-carbon atoms of a 2,5-diketopiperazine ring. The molecular architecture of stephacidin B, formally a dimer of avrainvillamide, reveals a complex dimeric prenylated N-hydroxyindole alkaloid that contains 15 rings and 9 stereogenic centers and is one of the most complex indole alkaloids isolated from fungi. Stephacidin B rapidly converts into the electrophilic monomer avrainvillamide in cell culture, and there is evidence that the monomer avrainvillamide interacts with intracellular thiol-containing proteins, most likely by covalent modification.

(-)-Versicolamide B and (+)-Versicolamide B are spiroindole alkaloids isolated from the fungus Aspergillus that belong to a class of naturally occurring 2,5-diketopiperazines. The versicolamides are structurally complex spiro-cyclized versions of prenylated cyclo(L-Trp-L-Pro) derivatives which possess a unique spiro-fusion to a pyrrolidine at the 3-position of the oxindole core together with the bicyclo[2.2.2]diazaoctane ring system. While (-)-versicolamide B was isolated from the marine fungus Aspergillus sp. the enantiomer (+)-versicolamide B was isolated from the terrestrial fungi Aspergillus versicolor NRRL. The total asymmetric syntheses of both enantiomers have been achieved and the implications of their biosynthesis have been investigated.

Dideoxyverticillin A, also known as (+)-11,11′-dideoxyverticillin A, is a complex epipolythiodioxopiperazine initially isolated from the marine fungus Penicillium sp. in 1999. It has also been found in the marine fungus Bionectriaceae, and belongs to a class of naturally occurring 2,5-diketopiperazines.
Penicillium citrinum is an anamorph, mesophilic fungus species of the genus of Penicillium which produces tanzawaic acid A-D, ACC, Mevastatin, Quinocitrinine A, Quinocitrinine B, and nephrotoxic citrinin. Penicillium citrinum is often found on moldy citrus fruits and occasionally it occurs in tropical spices and cereals. This Penicillium species also causes mortality for the mosquito Culex quinquefasciatus. Because of its mesophilic character, Penicillium citrinum occurs worldwide. The first statin (Mevastatin) was 1970 isolated from this species.
Penicillium rugulosum is an anamorph species of fungus in the genus Penicillium which produces inulinase, luteoskyrin and (+) rugulosin.

Brevianamide F , also known as cyclo-(L-Trp-L-Pro), belongs to a class of naturally occurring 2,5-diketopiperazines. It is the simplest member and the biosynthetic precursor of a large family of biologically active prenylated tryptophan-proline 2,5-diketopiperazines that are produced by the fungi A. fumigatus and Aspergillus sp. It has been isolated from the bacterium Streptomyces sp. strain TN58 and shown to possess activity against the Gram-positive bacteria S. aureus and Micrococcus luteus. It has also been isolated from Bacillus cereus associated with the entomopathogenic nematode Rhabditis (Oscheius) sp. and shown to have antifungal activity against T. rubrum, C. neoformans, and C. albicans, better than amphotericin B. Although the proline 2,5-diketopiperazines are the most abundant and structurally diverse 2,5-diketopiperazines found in food, cyclo(L-Trp-L-Pro) has only been found as a minor 2,5-diketopiperazine (8.2 ppm) in autolyzed yeast extract. Initially, cyclo(L-Trp-L-Pro) and its DL, LD, and DD isomers showed potential for use in the treatment of cardiovascular dysfunction, but they were later shown to be hepatotoxic.

Bicyclomycin (Bicozamycin) is a broad spectrum antibiotic active against Gram-negative bacteria and the Gram-positive bacterium, Micrococcus luteus that was isolated from Streptomyces sapporonesis and Streptomyces aizumenses in 1972. It belongs to a class of naturally occurring 2,5-diketopiperazines, that are among the most numerous of all the naturally occurring peptide antibiotics. This clinically useful antibiotic is rapidly absorbed in humans when given intramuscularly, has low toxicity and has been used to treat diarrhea in humans and bacterial diarrhea in calves and pigs.

















