Fluoronitrobenzene may refer to three compounds with the formula FC6H4NO2:
Fluoronitrobenzene may refer to three compounds with the formula FC6H4NO2:
Compound may refer to:
German(s) may refer to:
Quinolone may refer to:
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Volatility or volatile may refer to:
Copper sulfate may refer to:
Sulfur oxide refers to many types of sulfur and oxygen containing compounds such as SO, SO2, SO3, S7O2, S6O2, S2O2, etc.
Iron sulfide or Iron sulphide can refer to range of chemical compounds composed of iron and sulfur.
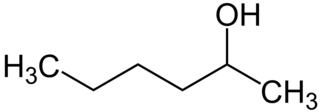
Hexanol may refer to any of the following isomeric organic compounds with the formula C6H13OH:
Linolenic acid is a type of naturally-occurring fatty acid. It can refer to either of two octadecatrienoic acids, or a mixture of the two. Linolenate is often found in vegetable oils; traditionally, such fatty acylates are reported as the fatty acids:
Butyne is an alkyne that contains 4 carbon and 6 hydrogen. It contains one triple bond and has two isomeric organic chemical compounds:
Pentyne may refer to:
Dioxin may refer to:

4-Nitrochlorobenzene is the organic compound with the formula ClC6H4NO2. It is a pale yellow solid. 4-Nitrochlorobenzene is a common intermediate in the production of a number of industrially useful compounds, including antioxidants commonly found in rubber. Other isomers with the formula ClC6H4NO2 include 2-nitrochlorobenzene and 3-nitrochlorobenzene.

2-Nitrochlorobenzene is an organic compound with the formula ClC6H4NO2. It is one of three isomeric nitrochlorobenzenes. It is a yellow crystalline solid that is important as a precursor to other compounds due to its two functional groups.
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
Halobenzenes are a group of aryl halides consisting of a benzene ring with halogen atoms as substituents.
In chemistry, the Halex process is used to convert aromatic chlorides to the corresponding aromatic fluorides. The process entails Halide exchange, hence the name. The reaction conditions call for hot solution of the aryl chloride and anhydrous potassium fluoride. Typical solvents are dimethylsulfoxide, dimethylformamide, and sulfolane. Potassium chloride is generated in the process. The reaction is mainly applied to nitro-substituted aryl chlorides.
4-Fluoronitrobenzene is an organic compound with the formula FC6H4NO2. It is one of three isomeric fluoronitrobenzenes. A yellow oil, it is prepared from 4-nitrochlorobenzene using the Halex process:
The molecular formula C6H4FNO2 may refer to: