Brass is an alloy of copper and zinc, in proportions which can be varied to achieve varying mechanical and electrical properties. It is a substitutional alloy: atoms of the two constituents may replace each other within the same crystal structure.

Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals and sometimes non-metals or metalloids such as arsenic, phosphorus or silicon. These additions produce a range of alloys that may be harder than copper alone, or have other useful properties, such as stiffness, ductility, or machinability.

Galvanization or galvanizing is the process of applying a protective zinc coating to steel or iron, to prevent rusting. The most common method is hot-dip galvanizing, in which the parts are submerged in a bath of molten hot zinc.
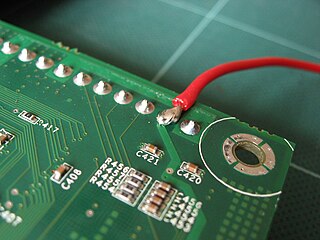
Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to adhere to and connect the pieces after cooling, which requires that an alloy suitable for use as solder have a lower melting point than the pieces being joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics.

Cosmetics comprise a range of products that are used to care for the face and body or to enhance or change the appearance of the face or body. The products include skin care, personal care, cosmetics and fragrance.

Hot-dip galvanization is a form of galvanization. It is the process of coating iron and steel with zinc, which alloys with the surface of the base metal when immersing the metal in a bath of molten zinc at a temperature of around 449 °C (840 °F). When exposed to the atmosphere, the pure zinc (Zn) reacts with oxygen (O2) to form zinc oxide (ZnO), which further reacts with carbon dioxide (CO2) to form zinc carbonate (ZnCO3), a usually dull grey, fairly strong material that protects the steel underneath from further corrosion in many circumstances. Galvanized steel is widely used in applications where corrosion resistance is needed without the cost of stainless steel, and is considered superior in terms of cost and life-cycle. It can be identified by the crystallization patterning on the surface (often called a "spangle").

Brazing is a metal-joining process in which two or more metal items are joined together by melting and flowing a filler metal into the joint, the filler metal having a lower melting point than the adjoining metal.

Tombac, as it is spelled in French, or tombak, is a brass alloy with high copper content and 5–20% zinc content. Tin, lead or arsenic may be added for colouration. It is a cheap malleable alloy mainly used for medals, ornament, decoration and some munitions. In older use, the term may apply to brass alloy with a zinc content as high as 28–35%.

Gun metal, also known as red brass in the United States, is a type of bronze – an alloy of copper, tin, and zinc. Proportions vary but 88% copper, 8–10% tin, and 2–4% zinc is an approximation. Originally used chiefly for making guns, it has largely been replaced by steel. Gunmetal, which casts and machines well and is resistant to corrosion from steam and salt water, is used to make steam and hydraulic castings, valves, gears, statues, and various small objects, such as buttons. It has a tensile strength of 221 to 310 MPa, a specific gravity of 8.7, a Brinell hardness of 65 to 74, and a melting point of around 1,000 degrees Celsius.
Extreme pressure additives, or EP additives, are additives for lubricants with a role to decrease wear of the parts of the gears exposed to very high pressures. They are also added to cutting fluids for machining of metals.
Painterwork is a specific painting technique, stemming from when paints were not commercially available as today.

Came glasswork is the process of joining cut pieces of art glass through the use of came strips or foil into picturesque designs in a framework of soldered metal.

Spelter is a zinc–lead alloy that ages to resemble bronze, but is softer and has a lower melting point. The name can also refer to a copper–zinc alloy used for brazing, or to pure zinc.

Eye shadow is a cosmetic that is applied on the eyelids and under the eyes. It is commonly used to make the wearer's eyes stand out or look more attractive.

Carbonate-hosted lead-zinc ore deposits are important and highly valuable concentrations of lead and zinc sulfide ores hosted within carbonate formations and which share a common genetic origin.

Metals used for architectural purposes include lead, for water pipes, roofing, and windows; tin, formed into tinplate; zinc, copper and aluminium, in a range of applications including roofing and decoration; and iron, which has structural and other uses in the form of cast iron or wrought iron, or made into steel. Metal alloys used in building include bronze ; brass ; monel metal and nickel silver, mainly consisting of nickel and copper; and stainless steel, with important components of nickel and chromium.

Soldering is a process in which two or more items are joined together by melting and putting a filler metal (solder) into the joint, the filler metal having a lower melting point than the adjoining metal. Unlike welding, soldering does not involve melting the work pieces. In brazing, the work piece metal also does not melt, but the filler metal is one that melts at a higher temperature than in soldering. In the past, nearly all solders contained lead, but environmental and health concerns have increasingly dictated use of lead-free alloys for electronics and plumbing purposes.