Gadolinium iodide may refer to:
- Gadolinium diiodide, GdI2
- Gadolinium(III) iodide (gadolinium triiodide), GdI3
Gadolinium iodide may refer to:

Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of 20 °C (68 °F) is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties.
Naturally occurring gadolinium (64Gd) is composed of 6 stable isotopes, 154Gd, 155Gd, 156Gd, 157Gd, 158Gd and 160Gd, and 1 radioisotope, 152Gd, with 158Gd being the most abundant (24.84% natural abundance). The predicted double beta decay of 160Gd has never been observed; only a lower limit on its half-life of more than 1.3×1021 years has been set experimentally.

Gadolinium(III) chloride, also known as gadolinium trichloride, is GdCl3. It is a colorless, hygroscopic, water-soluble solid. The hexahydrate GdCl3∙6H2O is commonly encountered and is sometimes also called gadolinium trichloride. Gd3+ species are of special interest because the ion has the maximum number of unpaired spins possible, at least for known elements. With seven valence electrons and seven available f-orbitals, all seven electrons are unpaired and symmetrically arranged around the metal. The high magnetism and high symmetry combine to make Gd3+ a useful component in NMR spectroscopy and MRI.
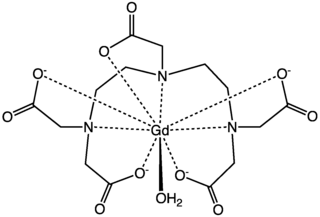
Gadopentetic acid, sold under the brand name Magnevist, is a gadolinium-based MRI contrast agent.
GD, Gd, or gd may refer to:

Gadolinium(III) oxide (archaically gadolinia) is an inorganic compound with the formula Gd2O3. It is one of the most commonly available forms of the rare-earth element gadolinium, derivatives of which are potential contrast agents for magnetic resonance imaging.
Gadolinium oxysulfide (Gd2O2S), also called gadolinium sulfoxylate, GOS or Gadox, is an inorganic compound, a mixed oxide-sulfide of gadolinium.

Gadolinium(III) nitrate is an inorganic compound of gadolinium. This salt is used as a water-soluble neutron poison in nuclear reactors. Gadolinium nitrate, like all nitrate salts, is an oxidizing agent.
MRI contrast agents are contrast agents used to improve the visibility of internal body structures in magnetic resonance imaging (MRI). The most commonly used compounds for contrast enhancement are gadolinium-based. Such MRI contrast agents shorten the relaxation times of nuclei within body tissues following oral or intravenous administration.

Perfusion MRI or perfusion-weighted imaging (PWI) is perfusion scanning by the use of a particular MRI sequence. The acquired data are then post-processed to obtain perfusion maps with different parameters, such as BV, BF, MTT and TTP.
Gadolinium(III) bromide is a crystalline compound of gadolinium atoms and three bromine atoms. This salt is hygroscopic.
Gadolinium(III) fluoride is an inorganic compound with a chemical formula GdF3.
Gadolinium phosphide is an inorganic compound of gadolinium and phosphorus with the chemical formula GdP.
Neodymium(III) iodide is an inorganic salt of iodine and neodymium the formula NdI3. Neodymium uses the +3 oxidation state in the compound. The anydrous compound is a green powdery solid at room temperature.
Gadolinium(III) hydroxide is a chemical compound with the formula Gd(OH)3. Its nanoparticles has a potential use for layering various drugs, such as diclofenac, ibuprofen, and naproxen.
Carbide iodides are mixed anion compounds containing iodide and carbide anions. Many carbide iodides are cluster compounds, containing one, two or more carbon atoms in a core, surrounded by a layer of metal atoms, encased in a shell of iodide ions. These ions may be shared between clusters to form chains, double chains or layers.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Gadolinium(III) iodide is an iodide of gadolinium, with the chemical formula of GdI3. It is a yellow, highly hygroscopic solid with a bismuth(III) iodide-type crystal structure. In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.

Gadolinium diiodide is an inorganic compound, with the chemical formula of GdI2. It is an electride, with the ionic formula of Gd3+(I−)2e−, and therefore not a true gadolinium(II) compound. It is ferromagnetic at 276 K with a saturation magnetization of 7.3 B; it exhibits a large negative magnetoresistance (~70%) at 7 T near room temperature. It can be obtained by reacting gadolinium and gadolinium(III) iodide at a high temperature: