Related Research Articles
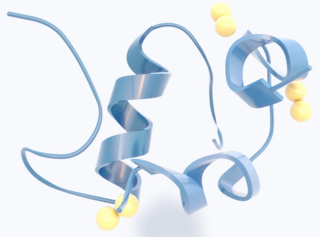
Insulin is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (INS) gene. It is the main anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats, and protein by promoting the absorption of glucose from the blood into cells of the liver, fat, and skeletal muscles. In these tissues the absorbed glucose is converted into either glycogen, via glycogenesis, or fats (triglycerides), via lipogenesis; in the liver, glucose is converted into both. Glucose production and secretion by the liver are strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is thus an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules in the cells. Low insulin in the blood has the opposite effect, promoting widespread catabolism, especially of reserve body fat.
Insulin resistance (IR) is a pathological condition in which cells either fail to respond normally to the hormone insulin or downregulate insulin receptors in response to hyperinsulinemia.
The following is a glossary of diabetes which explains terms connected with diabetes.

Diabetic ketoacidosis (DKA) is a potentially life-threatening complication of diabetes mellitus. Signs and symptoms may include vomiting, abdominal pain, deep gasping breathing, increased urination, weakness, confusion and occasionally loss of consciousness. A person's breath may develop a specific "fruity" smell. The onset of symptoms is usually rapid. People without a previous diagnosis of diabetes may develop DKA as the first obvious symptom.

Beta cells (β-cells) are specialized endocrine cells located within the pancreatic islets of Langerhans responsible for the production and release of insulin and amylin. Constituting ~50–70% of cells in human islets, beta cells play a vital role in maintaining blood glucose levels. Problems with beta cells can lead to disorders such as diabetes.

The glucose tolerance test is a medical test in which glucose is given and blood samples taken afterward to determine how quickly it is cleared from the blood. The test is usually used to test for diabetes, insulin resistance, impaired beta cell function, and sometimes reactive hypoglycemia and acromegaly, or rarer disorders of carbohydrate metabolism. In the most commonly performed version of the test, an oral glucose tolerance test (OGTT), a standard dose of glucose is ingested by mouth and blood levels are checked two hours later. Many variations of the GTT have been devised over the years for various purposes, with different standard doses of glucose, different routes of administration, different intervals and durations of sampling, and various substances measured in addition to blood glucose.
Hyperglycemia is a condition in which an excessive amount of glucose circulates in the blood plasma. This is generally a blood sugar level higher than 11.1 mmol/L (200 mg/dL), but symptoms may not start to become noticeable until even higher values such as 13.9–16.7 mmol/L (~250–300 mg/dL). A subject with a consistent fasting blood glucose range between ~5.6 and ~7 mmol/L is considered slightly hyperglycemic, and above 7 mmol/L is generally held to have diabetes. For diabetics, glucose levels that are considered to be too hyperglycemic can vary from person to person, mainly due to the person's renal threshold of glucose and overall glucose tolerance. On average, however, chronic levels above 10–12 mmol/L (180–216 mg/dL) can produce noticeable organ damage over time.

The blood sugar level, blood sugar concentration, blood glucose level, or glycemia is the measure of glucose concentrated in the blood. The body tightly regulates blood glucose levels as a part of metabolic homeostasis.
Hyperinsulinemic hypoglycemia describes the condition and effects of low blood glucose caused by excessive insulin. Hypoglycemia due to excess insulin is the most common type of serious hypoglycemia. It can be due to endogenous or injected insulin.

Hyperinsulinism refers to an above normal level of insulin in the blood of a person or animal. Normal insulin secretion and blood levels are closely related to the level of glucose in the blood, so that a given level of insulin can be normal for one blood glucose level but low or high for another. Hyperinsulinism can be associated with several types of medical problems, which can be roughly divided into two broad and largely non-overlapping categories: those tending toward reduced sensitivity to insulin and high blood glucose levels (hyperglycemia), and those tending toward excessive insulin secretion and low glucose levels (hypoglycemia).

Hyperinsulinemia is a condition in which there are excess levels of insulin circulating in the blood relative to the level of glucose. While it is often mistaken for diabetes or hyperglycaemia, hyperinsulinemia can result from a variety of metabolic diseases and conditions, as well as non-nutritive sugars in the diet. While hyperinsulinemia is often seen in people with early stage type 2 diabetes mellitus, it is not the cause of the condition and is only one symptom of the disease. Type 1 diabetes only occurs when pancreatic beta-cell function is impaired. Hyperinsulinemia can be seen in a variety of conditions including diabetes mellitus type 2, in neonates and in drug-induced hyperinsulinemia. It can also occur in congenital hyperinsulinism, including nesidioblastosis.
The homeostatic model assessment (HOMA) is a method used to quantify insulin resistance and beta-cell function. It was first described under the name HOMA by Matthews et al. in 1985.
The quantitative insulin sensitivity check index (QUICKI) is derived using the inverse of the sum of the logarithms of the fasting insulin and fasting glucose:
Hyperosmolar hyperglycemic state (HHS), also known as hyperosmolar non-ketotic state (HONK), is a complication of diabetes mellitus in which high blood sugar results in high osmolarity without significant ketoacidosis. Symptoms include signs of dehydration, weakness, leg cramps, vision problems, and an altered level of consciousness. Onset is typically over days to weeks. Complications may include seizures, disseminated intravascular coagulopathy, mesenteric artery occlusion, or rhabdomyolysis.
Pulsatile intravenous insulin therapy, sometimes called metabolic activation therapy or cellular activation therapy, describes in a literal sense the intravenous injection of insulin in pulses versus continuous infusions. Injection of insulin in pulses mimics the physiological secretions of insulin by the pancreas into the portal vein which then drains into the liver. In healthy, non-diabetic individuals, pancreatic secretions of insulin correspond to the intake of food. The pancreas will secrete variable amounts of insulin based upon the amount of food consumed among other factors. Continuous exposure to insulin and glucagon is known to decrease the hormones' metabolic effectiveness on glucose production in humans due to the body developing an increased tolerance to the hormones. Down-regulation at the cellular level may partially explain the decreased action of steady-state levels of insulin, while pulsatile hormone secretion may allow recovery of receptor affinity and numbers for insulin. Intermittent intravenous insulin administration with peaks of insulin concentrations may enhance suppression of gluconeogenesis and reduce hepatic glucose production.

Blood sugar regulation is the process by which the levels of blood sugar, the common name for glucose dissolved in blood plasma, are maintained by the body within a narrow range.
Mladen Vranic, MD, DSc, O.C., O.Ont, FRSC, FRCP(C), FCAHS, Canadian Medical Hall of Fame[CMHF] April 3, 1930 – June 18, 2019, was a Croatian-born diabetes researcher, best known for his work in tracer methodology, exercise and stress in diabetes, the metabolic effects of hormonal interactions, glucagon physiology, extrapancreatic glucagon, the role of the direct and indirect metabolic effects of insulin and the prevention of hypoglycemia. Vranic was recognized by a number of national and international awards for his research contributions, mentoring and administration including the Orders of Canada (Officer) and Ontario.

The Disposition index (DI) is a measure for the loop gain of the insulin-glucose feedback control system. It is defined as the product of insulin sensitivity times the amount of insulin secreted in response to blood glucose levels. "Metabolically healthy" Insulin resistant individuals can maintain normal responses to blood glucose due to the fact that higher levels of insulin are secreted as long as the beta cells of the pancreas are able to increase their output of insulin to compensate for the insulin resistance. But the ratio of the incremental increase in plasma insulin associated with an incremental increase in plasma glucose provides a better measure of beta cell function than the plasma insulin response to a glucose challenge. Loss of function of the beta cells, reducing their capacity to compensate for insulin resistance, results in a lower disposition index.
The Metabolic Score for Insulin Resistance (METS-IR) is a metabolic index developed with the aim to quantify peripheral insulin sensitivity in humans; it was first described under the name METS-IR by Bello-Chavolla et al. in 2018. It was developed by the Metabolic Research Disease Unit at the Instituto Nacional de Ciencias Médicas Salvador Zubirán and validated against the euglycemic hyperinsulinemic clamp and the frequently-sampled intravenous glucose tolerance test in Mexican population. It is a non-insulin-based alternative to insulin-based methods to quantify peripheral insulin sensitivity and an alternative to SPINA Carb, the Homeostatic Model Assessment (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI). METS-IR is currently validated for its use to assess cardio-metabolic risk in Latino population.
Pancreatic beta cell function is one of the preconditions of euglycaemia, i.e. normal blood sugar regulation. It is defined as insulin secretory capacity, i.e. the maximum amount of insulin to be produced by beta cells in a given unit of time.
References
- ↑ Linda von Wartburg, “What's a Glucose Clamp, Anyway?” Diabetes Health. Nov 7, 2007. Archived 2010-01-31 at the Wayback Machine
- ↑ DeFronzo RA, Tobin JD, Andres R. “Glucose Clamp Technique: a Method for Quantifying Insulin Secretion and Resistance.” Am. J. Physiol. 1979, 237, E214–E223.
- ↑ Marcus Hompesch and Klaus Rave. “An Analysis of How to Measure Glucose during Glucose Clamps: Are Glucose Meters Ready for Research?” J. Diabetes Sci. Technol. 2008, 2, 896–898.
- ↑ GmbH, Profil Institut für Stoffwechselforschung. "ClampArt and other glucose clamps at Profil". www.profil.com. Retrieved 2015-09-24.