Related Research Articles

Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies. It is mainly used as a pain medication, and is also commonly used recreationally, or to make other illicit opioids. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle; by injection under the skin; intravenously; injection into the space around the spinal cord; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are available as MS-Contin, Kadian, and other brand names as well as generically.
Endorphins are chemical signals in the brain that block the perception of pain and increase feelings of wellbeing. They are produced and stored in an area of the brain known as the pituitary gland.
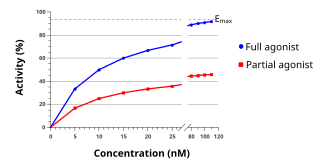
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.

Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, reversing opioid overdose, and suppressing cough. Extremely potent opioids such as carfentanil are approved only for veterinary use. Opioids are also frequently used non-medically for their euphoric effects or to prevent withdrawal. Opioids can cause death and have been used for executions in the United States.
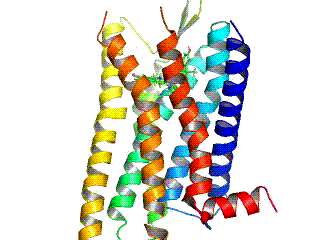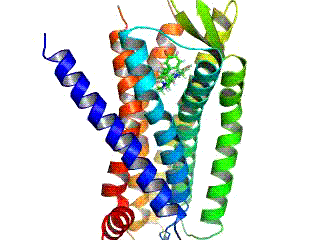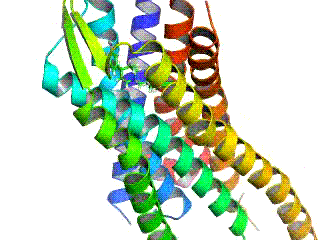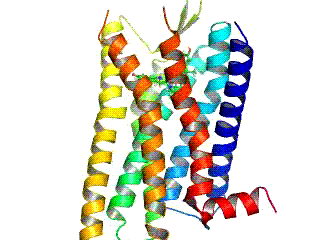
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract.
Agmatine, also known as 4-aminobutyl-guanidine, was discovered in 1910 by Albrecht Kossel. It is a chemical substance which is naturally created from the amino acid arginine. Agmatine has been shown to exert modulatory action at multiple molecular targets, notably: neurotransmitter systems, ion channels, nitric oxide (NO) synthesis and polyamine metabolism and this provides bases for further research into potential applications.
Cross-tolerance is a phenomenon that occurs when tolerance to the effects of a certain drug produces tolerance to another drug. It often happens between two drugs with similar functions or effects—for example, acting on the same cell receptor or affecting the transmission of certain neurotransmitters. Cross-tolerance has been observed with pharmaceutical drugs such as anti-anxiety agents and illicit substances, and sometimes the two of them together. Often, a person who uses one drug can be tolerant to a drug that has a completely different function. This phenomenon allows one to become tolerant to a drug that they have never used before.

Nociceptin/orphanin FQ (N/OFQ), a 17-amino acid neuropeptide, is the endogenous ligand for the nociceptin receptor. Nociceptin acts as a potent anti-analgesic, effectively counteracting the effect of pain-relievers; it's activation is associated with brain functions such as pain sensation and fear learning.

Hans Walter Kosterlitz FRS was a German-born British biochemist.

Endomorphins are considered to be natural opioid neurotransmitters central to pain relief. The two known endomorphins, endomorphin-1 and endomorphin-2, are tetrapeptides, consisting of Tyr-Pro-Trp-Phe and Tyr-Pro-Phe-Phe amino acid sequences respectively. These sequences fold into tertiary structures with high specificity and affinity for the μ-opioid receptor, binding it exclusively and strongly. Bound μ-opioid receptors typically induce inhibitory effects on neuronal activity. Endomorphin-like immunoreactivity exists within the central and peripheral nervous systems, where endomorphin-1 appears to be concentrated in the brain and upper brainstem, and endomorphin-2 in the spinal cord and lower brainstem. Because endomorphins activate the μ-opioid receptor, which is the target receptor of morphine and its derivatives, endomorphins possess significant potential as analgesics with reduced side effects and risk of addiction.
Opioid-induced hyperalgesia (OIH) or opioid-induced abnormal pain sensitivity, also called paradoxical hyperalgesia, is an uncommon condition of generalized pain caused by the long-term use of high dosages of opioids such as morphine, oxycodone, and methadone. OIH is not necessarily confined to the original affected site. This means that if the person was originally taking opioids due to lower back pain, when OIH appears, the person may experience pain in the entire body, instead of just in the lower back. Over time, individuals taking opioids can also develop an increasing sensitivity to noxious stimuli, even evolving a painful response to previously non-noxious stimuli (allodynia). This means that if the person originally felt pain from twisting or from sitting too long, the person might now additionally experience pain from a light touch or from raindrops falling on the skin.

The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(mu)-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium and for which the receptor was named, with mu being the Greek letter m. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, inhibiting adenylate cyclase activity, lowering cAMP levels.

The nociceptin opioid peptide receptor (NOP), also known as the nociceptin/orphanin FQ (N/OFQ) receptor or kappa-type 3 opioid receptor, is a protein that in humans is encoded by the OPRL1 gene. The nociceptin receptor is a member of the opioid subfamily of G protein-coupled receptors whose natural ligand is the 17 amino acid neuropeptide known as nociceptin (N/OFQ). This receptor is involved in the regulation of numerous brain activities, particularly instinctive and emotional behaviors. Antagonists targeting NOP are under investigation for their role as treatments for depression and Parkinson's disease, whereas NOP agonists have been shown to act as powerful, non-addictive painkillers in non-human primates.
Leu-enkephalin is an endogenous opioid peptide neurotransmitter with the amino acid sequence Tyr-Gly-Gly-Phe-Leu that is found naturally in the brains of many animals, including humans. It is one of the two forms of enkephalin; the other is met-enkephalin. The tyrosine residue at position 1 is thought to be analogous to the 3-hydroxyl group on morphine. Leu-enkephalin has agonistic actions at both the μ- and δ-opioid receptors, with significantly greater preference for the latter. It has little to no effect on the κ-opioid receptor.

Proglumide (Milid) is a drug that inhibits gastrointestinal motility and reduces gastric secretions. It acts as a cholecystokinin antagonist, which blocks both the CCKA and CCKB subtypes. It was used mainly in the treatment of stomach ulcers, although it has now been largely replaced by newer drugs for this application.
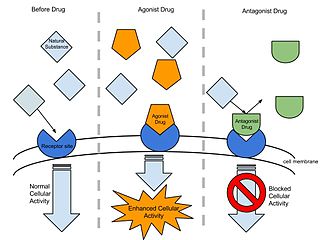
In pharmacology the term agonist-antagonist or mixed agonist/antagonist is used to refer to a drug which under some conditions behaves as an agonist while under other conditions, behaves as an antagonist.

CI-988 (PD-134,308) is a drug which acts as a cholecystokinin antagonist, selective for the CCKB subtype. In animal studies it showed anxiolytic effects and potentiated the analgesic action of both morphine and endogenous opioid peptides, as well as preventing the development of tolerance to opioids and reducing symptoms of withdrawal. Consequently, it was hoped that it might have clinical applications for the treatment of pain and anxiety in humans, but trial results were disappointing with only minimal therapeutic effects observed even at high doses. The reason for the failure of CI-988 and other CCKB antagonists in humans despite their apparent promise in pre-clinical animal studies is unclear, although poor pharmacokinetic properties of the currently available drugs are a possible explanation, and CCKB antagonists are still being researched for possible uses as adjuvants to boost the activity of other drugs.

An opiate, in classical pharmacology, is a substance derived from opium. In more modern usage, the term opioid is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions with evidence of opiate trade and use for pain relief as early as the eighth century AD. Opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.
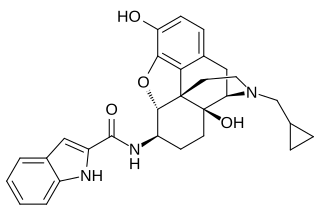
N-2′-Indolylnaltrexamine (INTA) is an opioid and derivative of β-naltrexamine. This molecule is loosely derived from the classical opioid morphine. This experimental drug candidate is under development as a κ-opioid receptor agonist for pain management with fewer adverse side effects. Preclinical study in mice showed potent analgesic effects with no tolerance or dependence. The mice also showed no adverse effects in the conditioned place aversion assay.
John Hughes is a British neuroscientist who shared the 1978 Albert Lasker Award for Basic Medical Research for the discovery of met-enkephalin and leu-enkephalin. This discovery demonstrated that opiate drugs exert their effects on the human brain by mimicking endogenous neurotransmitters, the opioid peptides.
References
- 1 2 3 "Professor Graeme Henderson". University of Bristol. Retrieved 24 July 2019.
- ↑ Henderson, G.; Hughes, J.; Kosterlitz, H.W. (1997). "A new example of a morphine-sensitive neuro-effector junction: Adrenergic transmission in the mouse vas deferens". British Journal of Pharmacology. 120: 393–395. doi:10.1111/j.1476-5381.1997.tb06820.x. PMC 3224316 .