
Grammotoxin is a toxin in the venom of the tarantula Grammostola spatulata . It is a protein toxin that inhibits P-, Q- and N-type voltage-gated calcium channels (Ca 2+ channels) in neurons. Grammotoxin is also known as omega-grammotoxin SIA.

Grammotoxin is a toxin in the venom of the tarantula Grammostola spatulata . It is a protein toxin that inhibits P-, Q- and N-type voltage-gated calcium channels (Ca 2+ channels) in neurons. Grammotoxin is also known as omega-grammotoxin SIA.
Grammotoxin is a 36 Amino Acid protein toxin, which has the following sequence: Asp-Cys-Val-Arg-Phe-Trp-Gly-Lys-Cys-Ser-Gln-Thr-Ser-Asp-Cys-Cys-Pro-His-Leu-Ala-Cys-Lys-Ser-Lys-Trp-Pro-Arg-Asn-Ile-Cys-Val-Trp-Asp-Gly-Ser-Val (DCVRFWGKCSQTSDCCPHLACKSKWPRNICVWDGSV).
It forms an inhibitor cystine knot motif, common in spider toxins. [1]
Its chemical formula is: C177H268N52O50S6 [2]
Grammotoxin can be purified from Grammostola spatulata venom by reverse phase high performance liquid chromatography. [3]
The toxin binding site on the channels has high affinity for the toxins when they are closed and low affinity when channels are activated. [4] As a result, the toxin preferentially binds to the closed channels. It binds at a region which contains the voltage-sensing domains. When bound, the toxin makes it more difficult for channels to be opened by depolarization, so much larger depolarizations are required for channel activation. [4] Grammotoxin also binds to potassium channels but with lower affinity than to the calcium channels. [1]
Growth hormone–releasing hormone (GHRH), also known as somatocrinin or by several other names in its endogenous forms and as somatorelin (INN) in its pharmaceutical form, is a releasing hormone of growth hormone (GH). It is a 44-amino acid peptide hormone produced in the arcuate nucleus of the hypothalamus.

Delta atracotoxin is a low-molecular-weight neurotoxic polypeptide found in the venom of the Sydney funnel-web spider.
omega-Grammotoxin SIA (ω-grammotoxin SIA) is a protein toxin that inhibits P, Q, and N voltage-gated calcium channels (Ca2+ channels) in neurons.

Slotoxin is a peptide from Centruroides noxius Hoffmann scorpion venom. It belongs to the short scorpion toxin superfamily.

Margatoxin (MgTX) is a peptide that selectively inhibits Kv1.3 voltage-dependent potassium channels. It is found in the venom of Centruroides margaritatus, also known as the Central American Bark Scorpion. Margatoxin was first discovered in 1993. It was purified from scorpion venom and its amino acid sequence was determined.
Agitoxin is a toxin found in the venom of the scorpion Leiurus quinquestriatus hebraeus. Other toxins found in this species include charybdotoxin (CTX). CTX is a close homologue of Agitoxin.
The P-type calcium channel is a type of voltage-dependent calcium channel. Similar to many other high-voltage-gated calcium channels, the α1 subunit determines most of the channel's properties. The 'P' signifies cerebellar Purkinje cells, referring to the channel's initial site of discovery. P-type calcium channels play a similar role to the N-type calcium channel in neurotransmitter release at the presynaptic terminal and in neuronal integration in many neuronal types.

Spider toxins are a family of proteins produced by spiders which function as neurotoxins. The mechanism of many spider toxins is through blockage of calcium channels.
Cerastocytin is a thrombin-like serine protease in snake venom.

Tertiapin is a 21-amino acid peptide isolated from venom of the European honey bee. It blocks two different types of potassium channels, inward rectifier potassium channels (Kir) and calcium activated large conductance potassium channels (BK).
BeKm-1 is a toxin from the Central Asian scorpion Buthus eupeus. BeKm-1 acts by selectively inhibiting the human Ether-à-go-go Related Gene (hERG) channels, which are voltage gated potassium ion channels.

Guangxitoxin, also known as GxTX, is a peptide toxin found in the venom of the tarantula Plesiophrictus guangxiensis. It primarily inhibits outward voltage-gated Kv2.1 potassium channel currents, which are prominently expressed in pancreatic β-cells, thus increasing insulin secretion.
Hanatoxin is a toxin found in the venom of the Grammostola spatulata tarantula. The toxin is mostly known for inhibiting the activation of voltage-gated potassium channels, most specifically Kv4.2 and Kv2.1, by raising its activation threshold.
Hainantoxins (HNTX) are neurotoxins from the venom of the Chinese bird spider Haplopelma hainanum. Hainantoxins specifically inhibit tetrodotoxin-sensitive Voltage-gated sodium channels, thereby causing blockage of neuromuscular transmission and paralysis. Currently, 13 different hainantoxins are known, but only HNTX-I, -II, -III, -IV and -V have been investigated in detail.
Huwentoxins (HWTX) are a group of neurotoxic peptides found in the venom of the Chinese bird spider Haplopelma schmidti. The species was formerly known as Haplopelma huwenum, Ornithoctonus huwena and Selenocosmia huwena. While structural similarity can be found among several of these toxins, HWTX as a group possess high functional diversity.
Agelenin, also called U1-agatoxin-Aop1a, is an antagonist of the presynaptic P-type calcium channel in insects. This neurotoxic peptide consists of 35 amino acids and can be isolated from the venom of the spider Allagelena opulenta.
CgNa is a peptide toxin isolated from the sea anemone Condylactis gigantea. It causes an increased action potential duration by slowing down the inactivation of tetrodotoxin-sensitive sodium channels.
Centruroides suffusus suffusus toxin II (CssII) is a scorpion β-toxin from the venom of the scorpion Centruroides suffusus suffusus. CssII primarily affects voltage-gated sodium channels by causing a hyperpolarizing shift of voltage dependence, a reduction in peak transient current, and the occurrence of resurgent currents.
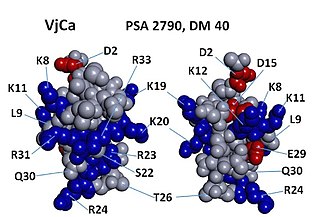
Vejocalcin (VjCa, also called Vejocalcine) is a toxin from the venom of the Mexican scorpion Vaejovis mexicanus. Vejocalcin is a member of the calcin family of toxins. It acts as a cell-penetrating peptide (CPP); it binds with high affinity and specificity to skeletal ryanodine receptor 1 (RYR1) of the sarcoplasmic reticulum, thereby triggering calcium release from intracellular Ca2+ stores.
LmαTX5 is an α-scorpion toxin which inhibits the fast inactivation of voltage-gated sodium channels. It has been identified through transcriptome analysis of the venom gland of Lychas mucronatus, also known as the Chinese swimming scorpion – a scorpion species which is widely distributed in Southeast Asia.