The actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinoid series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinoid chemistry to refer to any actinoid.
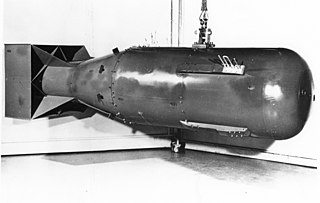
"Little Boy" was the codename for the type of atomic bomb dropped on the Japanese city of Hiroshima on 6 August 1945 during World War II. It was the first nuclear weapon used in warfare. The bomb was dropped by the Boeing B-29 Superfortress Enola Gay piloted by Colonel Paul W. Tibbets, Jr., commander of the 509th Composite Group of the United States Army Air Forces and Captain Robert A. Lewis. It exploded with an energy of approximately 15 kilotons of TNT (63 TJ) and caused widespread death and destruction throughout the city. The Hiroshima bombing was the second man-made nuclear explosion in history, after the Trinity test.

The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the U.S. Army Corps of Engineers. Nuclear physicist Robert Oppenheimer was the director of the Los Alamos Laboratory that designed the actual bombs. As engineer districts by convention carried the name of the city where they were located, the Army component of the project was designated the Manhattan District; Manhattan gradually superseded the official codename, Development of Substitute Materials, for the entire project. Along the way, the project absorbed its earlier British counterpart, Tube Alloys. The Manhattan Project began modestly in 1939, but grew to employ more than 130,000 people and cost nearly US$2 billion. Over 90 percent of the cost was for building factories and to produce fissile material, with less than 10 percent for development and production of the weapons. Research and production took place at more than thirty sites across the United States, the United Kingdom, and Canada.

In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into two or more smaller, lighter nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay.

Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) on exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are highly radioactive, with the most stable isotope being radium-226, which has a half-life of 1600 years and decays into radon gas (specifically the isotope radon-222). When radium decays, ionizing radiation is a product, which can excite fluorescent chemicals and cause radioluminescence.

Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.

Depleted uranium is uranium with a lower content of the fissile isotope U-235 than natural uranium. Natural uranium contains about 0.72% U-235, while the DU used by the U.S. Department of Defense contains 0.3% U-235 or less. The less radioactive and non-fissile uranium-238 constitutes the main component of depleted uranium. Uses of DU take advantage of its very high density of 19.1 g/cm3.
Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238, uranium-235, and uranium-234. 235U is the only nuclide existing in nature that is fissile with thermal neutrons.

Uraninite, formerly pitchblende, is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2, but due to oxidation the mineral typically contains variable proportions of U3O8. Additionally, due to radioactive decay, the ore also contains oxides of lead and trace amounts of helium. It may also contain thorium and rare earth elements.

In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay directly to a stable state, but rather undergo a series of decays until eventually a stable isotope is reached.

Uranium-235 (235U) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a fission chain reaction. It is the only fissile isotope that exists in nature as a primordial nuclide.

Uranium-238 is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.

Yellowcake is a type of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C.

Uranium glass is glass which has had uranium, usually in oxide diuranate form, added to a glass mix before melting for coloration. The proportion usually varies from trace levels to about 2% uranium by weight, although some 20th-century pieces were made with up to 25% uranium.
Uranium (92U) is a naturally occurring radioactive element that has no stable isotope. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in the Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from 214U to 242U. The standard atomic weight of natural uranium is 238.02891(3).
Natural uranium refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes from uranium-235, 48.6% from uranium-238, and 49.2% from uranium-234.

Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.

Uranium mining is the process of extraction of uranium ore from the ground. The worldwide production of uranium in 2019 amounted to 53,656 tonnes. Kazakhstan, Canada, and Australia were the top three producers and together account for 68% of world uranium production. Other important uranium producing countries in excess of 1,000 tonnes per year were Namibia, Niger, Russia, Uzbekistan and China. Uranium from mining is used almost entirely as fuel for nuclear power plants.
Uranium in the environment refers to the science of the sources, environmental behaviour, and effects of uranium on humans and other animals. Uranium is weakly radioactive and remains so because of its long physical half-life. The biological half-life for uranium is about 15 days. Normal functioning of the kidney, brain, liver, heart, and numerous other systems can be affected by uranium exposure, because uranium is a toxic metal. The use of depleted uranium (DU) in munitions is controversial because of questions about potential long-term health effects.

Uranium ore deposits are economically recoverable concentrations of uranium within the Earth's crust. Uranium is one of the more common elements in the Earth's crust, being 40 times more common than silver and 500 times more common than gold. It can be found almost everywhere in rock, soil, rivers, and oceans. The challenge for commercial uranium extraction is to find those areas where the concentrations are adequate to form an economically viable deposit. The primary use for uranium obtained from mining is in fuel for nuclear reactors.














