Related Research Articles
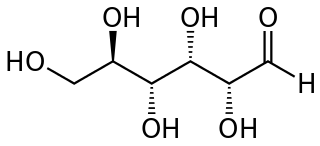
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid.

Molisch's test is a sensitive chemical test, named after Austrian botanist Hans Molisch, for the presence of carbohydrates, based on the dehydration of the carbohydrate by sulfuric acid or hydrochloric acid to produce an aldehyde, which condenses with two molecules of a phenol, resulting in a violet ring.
The oxidase test is used to determine whether an organism possesses the cytochrome c oxidase enzyme. The test is used as an aid for the differentiation of Neisseria, Moraxella, Campylobacter and Pasteurella species. It is also used to differentiate pseudomonads from related species.
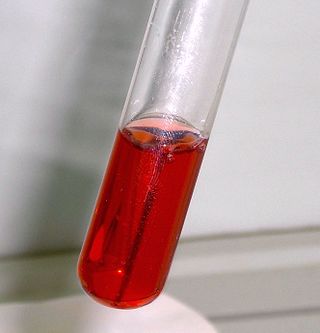
Seliwanoff’s test is a chemical test which distinguishes between aldose and ketose sugars. If the sugar contains a ketone group, it is a ketose. If a sugar contains an aldehyde group, it is an aldose. This test relies on the principle that, when heated, ketoses are more rapidly dehydrated than aldoses. It is named after Theodor Seliwanoff, the chemist who devised the test. When added to a solution containing ketoses, a red color is formed rapidly indicating a positive test. When added to a solution containing aldoses, a slower forming light pink is observed instead.
The Duquenois reagent used in the Rapid Modified Duquenois–Levine test, is an established screening test for the presence of cannabis. The test was initially developed in the 1930s by the French Medical Biochemist, Pierre Duquénois (1904–1986), and was adopted in the 1950s by the United Nations as the preferred test for cannabis, and originally claimed to be specific to cannabis.

Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride with the chemical formula HCl(aq). It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical.
The Trinder spot test is a diagnostic test used in medicine to determine exposure to salicylates, particularly to salicylic acid. The test employs the Trinder reagent which is mixed with a patient's urine. The colour change, resulting from the Trinder reaction, is immediate, enabling rapid bedside assessment.

The xanthoproteic reaction is a method that can be used to detect a presence of protein soluble in a solution, using concentrated nitric acid. The test gives a positive result in amino acids carrying aromatic groups, especially in the presence of tyrosine. If the test is positive the proof is neutralized with an alkali, turning dark yellow. The yellow colour is due to xanthoproteic acid which is formed due to nitration of certain amino acids, most common examples being tyrosine and tryptophan. This chemical reaction is a qualitative test, determining the presence or absence of proteins.

The Sakaguchi test is a chemical test used to detect presence of arginine in proteins. It is named after the Japanese food scientist and organic chemist, Shoyo Sakaguchi (1900–1995) who described the test in 1925. The Sakaguchi reagent used in the test consists of 1-Naphthol and a drop of sodium hypobromite. The guanidino (–C group in arginine reacts with the Sakaguchi reagent to form a red-coloured complex.
The Hopkins-Cole reaction, also known as the glyoxylic acid reaction, is a chemical test used for detecting the presence of tryptophan in proteins. A protein solution is mixed with Hopkins Cole reagent, which consists of glyoxylic acid. Concentrated sulfuric acid is slowly added to form two layers. A purple ring appears between the two layers if the test is positive for tryptophan. Nitrites, chlorates, nitrates and excess chlorides prevent the reaction from occurring.
The Sullivan reaction is a chemical test used for detecting the presence of cysteine or cystine in proteins. A red colour appears when a protein with cysteine or cystine is heated with sodium 1,2-naphthoquinone-4-sulfonate and sodium dithionite under alkaline conditions. This was pioneered by the American organic and industrial chemist Eugene Cornelius Sullivan (1872–1962).
Nylander's test is a chemical test used for detecting the presence of reducing sugars. Glucose or fructose reduces bismuth oxynitrate to bismuth under alkaline conditions. When Nylander's reagent, which consists of bismuth nitrate, potassium sodium tartrate and potassium hydroxide, is added to a solution with reducing sugars, a black precipitate of metallic bismuth is formed.
The rapid furfural test is a chemical test used to distinguish between glucose and fructose. The rapid furfural test is similar to Molisch's test but uses concentrated hydrochloric acid instead of concentrated sulfuric acid and the solution is boiled. Dilute sugar solution is added to ethanolic 1-naphthol and concentrated hydrochloric acid. The solution is then boiled and if a purple colour forms within thirty seconds, fructose is present. If a purple colour does not appear before thirty seconds, glucose is present.
Heller's test is a chemical test that shows that strong acids cause the denaturation of precipitated proteins. Concentrated nitric acid is added to a protein solution from the side of the test tube to form two layers. A white ring appears between the two layers if the test is positive. Heller's test is commonly used to test for the presence of proteins in urine. This test was discovered by the Austrian Chemist, Johann Florian Heller (1813-1871).
Kelling's test is a chemical test used for detecting the presence of lactic acid in gastric juice.
Gmelin's test is a chemical test used for detecting the presence of bile pigments in urine. It is named after Leopold Gmelin, who introduced the test. Five millilitres of urine is slowly added to five millilitres of concentrated nitric acid in a test-tube. Different coloured rings between the two layers are visible if bile pigments are present as they are oxidised to various chemical products. Nitric acid is used as the oxidising agent. Blue, green and violet rings are seen if bilirubin is present. Gmelin's test is not sensitive so a positive result always indicates the presence of bile pigments but a negative result does not exclude the presence of small quantities of bile pigments.
Hay's test, also known as Hay's sulphur powder test, is a chemical test used for detecting the presence of bile salts in urine.
The murexide test is an analytical technique to identify the presence of caffeine and other purine derivatives in a sample. These compounds do not respond to the common alkaloid detecting tests such as Dragendorff's reagent. In this test the alkaloids are mixed with a tiny amount of potassium chlorate and a drop of hydrochloric acid. The sample is then evaporated to dryness and the resulting residue is exposed to ammonia vapour. Purine alkaloids produce a pinkish-purple color in this test. Murexide with a purple color is also produced in this test.
A spot test in lichenology is a spot analysis used to help identify lichens. It is performed by placing a drop of a chemical on different parts of the lichen and noting the colour change associated with application of the chemical. The tests are routinely encountered in dichotomous keys for lichen species, and they take advantage of the wide array of lichen products produced by lichens and their uniqueness among taxa. As such, spot tests reveal the presence or absence of chemicals in various parts of a lichen. They were first proposed by the botanist William Nylander in 1866.
Salkowski's test, also known simply as Salkowski test, is a qualitative chemical test, that is used in chemistry and biochemistry for detecting a presence of cholesterol and other sterols. This biochemical method got its name after German biochemist Ernst Leopold Salkowski, who is known for development of multiple new chemical tests, that are used for detection of different kinds of molecules. A solution that has tested positive on the Salkowski's test becomes red and gets yellow glow.
References
- ↑ Chary (1 January 2004). Practical Biochemistry for Medical and Dental Students. Jaypee Brothers Publishers. p. 26. ISBN 978-81-8061-233-6.
- ↑ Shankara (1 December 2008). Practical Biochemistry 2008. Jaypee Brothers Publishers. p. 48. ISBN 978-81-8448-259-1.
- ↑ G. P. TALWAR; L .M. SRIVASTAVA (1 January 2002). TEXTBOOK OF BIOCHEMISTRY AND HUMAN BIOLOGY. PHI Learning Pvt. Ltd. p. 620. ISBN 978-81-203-1965-3.