
In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.
Benedict's reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate. It is often used in place of Fehling's solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive result. Such tests that use this reagent are called the Benedict's tests. A positive test with Benedict's reagent is shown by a color change from clear blue to brick-red with a precipitate.

In organic chemistry, Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone functional groups, and as a test for reducing sugars and non-reducing sugars, supplementary to the Tollens' reagent test. The test was developed by German chemist Hermann von Fehling in 1849.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Francois Auguste Victor Grignard was a French chemist who won the Nobel Prize for his discovery of the eponymously named Grignard reagent and Grignard reaction, both of which are important in the formation of carbon–carbon bonds. He also wrote some of his experiments in his laboratory notebooks.
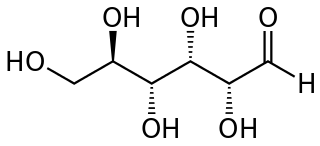
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.

The bicinchoninic acid assay, also known as the Smith assay, after its inventor, Paul K. Smith at the Pierce Chemical Company, now part of Thermo Fisher Scientific, is a biochemical assay for determining the total concentration of protein in a solution, similar to Lowry protein assay, Bradford protein assay or biuret reagent. The total protein concentration is exhibited by a color change of the sample solution from blue to purple in proportion to protein concentration, which can then be measured using colorimetric techniques. The BCA assay was patented by Pierce Chemical Company in 1989 & the patent expired in 2006.
The Lowry protein assay is a biochemical assay for determining the total level of protein in a solution. The total protein concentration is exhibited by a color change of the sample solution in proportion to protein concentration, which can then be measured using colorimetric techniques. It is named for the biochemist Oliver H. Lowry who developed the reagent in the 1940s. His 1951 paper describing the technique is the most-highly cited paper ever in the scientific literature, cited over 300,000 times.

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.
The Carius halogen method in analytical chemistry is a method for the quantitative determination of halogens in chemical substances.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.
In analytical chemistry, quantitative analysis is the determination of the absolute or relative abundance of one, several or all particular substance(s) present in a sample. It relates to the determination of percentage of constituents in any give sample.
Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule.

Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. It is a name reaction, named for Kazuhiko Takai, who first reported it in 1986. In the original reaction, the organochromium species is generated from iodoform or bromoform and an excess of chromium(II) chloride and the product is a vinyl halide. One main advantage of this reaction is the E-configuration of the double bond that is formed. According to the original report, existing alternatives such as the Wittig reaction only gave mixtures.
The ferric chloride test is used to determine the presence of phenols in a given sample or compound. Enols, hydroxamic acids, oximes, and sulfinic acids give positive results as well. The bromine test is useful to confirm the result, although modern spectroscopic techniques are far superior in determining the identity of the unknown. The quantity of total phenols may be spectroscopically determined by the Folin–Ciocalteau assay.

In chemistry, the haloform reaction is a chemical reaction in which a haloform is produced by the exhaustive halogenation of an acetyl group, in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform, bromoform, or iodoform. Note that fluoroform can't be prepared in this way.

A urine test strip or dipstick is a basic diagnostic tool used to determine pathological changes in a patient's urine in standard urinalysis.

Reductions with samarium(II) iodide involve the conversion of various classes of organic compounds into reduced products through the action of samarium(II) iodide, a mild one-electron reducing agent.














