Gram stain, is a method of staining used to classify bacterial species into two large groups: gram-positive bacteria and gram-negative bacteria. It may also be used to diagnose a fungal infection. The name comes from the Danish bacteriologist Hans Christian Gram, who developed the technique in 1884.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

Sulfites or sulphites are compounds that contain the sulfite ion, SO2−
3. The sulfite ion is the conjugate base of bisulfite. Although its acid is elusive, its salts are widely used.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
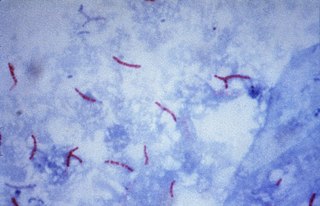
The Ziehl-Neelsen stain, also known as the acid-fast stain, is a bacteriological staining technique used in cytopathology and microbiology to identify acid-fast bacteria under microscopy, particularly members of the Mycobacterium genus. This staining method was initially introduced by Paul Ehrlich (1854–1915) and subsequently modified by the German bacteriologists Franz Ziehl (1859–1926) and Friedrich Neelsen (1854–1898) during the late 19th century.
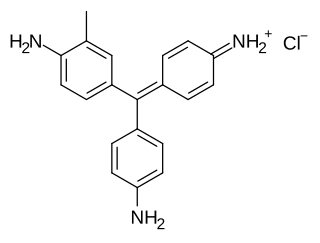
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl. There are other similar chemical formulations of products sold as fuchsine, and several dozen other synonyms of this molecule.

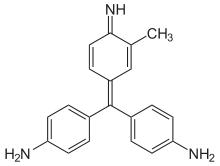
New fuchsine is an organic compound with the formula [(H2N(CH3)C6H3)3C]Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as single dyes. The other is pararosaniline. It is prepared by condensation of ortho-toluidine with formaldehyde. This process initially gives the benzhydrol 4,4'-bis(dimethylamino)benzhydrol, which is further condensed to give the leuco (colorless) tertiary alcohol [(H2N(CH3)C6H3)3COH, which is oxidized in acid to give the dye.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.

In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

Periodic acid–Schiff (PAS) is a staining method used to detect polysaccharides such as glycogen, and mucosubstances such as glycoproteins, glycolipids and mucins in tissues. The reaction of periodic acid oxidizes the vicinal diols in these sugars, usually breaking up the bond between two adjacent carbons not involved in the glycosidic linkage or ring closure in the ring of the monosaccharide units that are parts of the long polysaccharides, and creating a pair of aldehydes at the two free tips of each broken monosaccharide ring. The oxidation condition has to be sufficiently regulated so as to not oxidize the aldehydes further. These aldehydes then react with the Schiff reagent to give a purple-magenta color. A suitable basic stain is often used as a counterstain.

Sodium dithionite is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions.

The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion HSO−
3. Salts containing the HSO−
3 ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+HSO−
3.
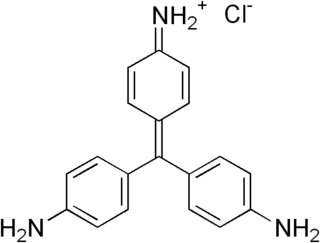
Pararosaniline, Basic Red 9, or C.I. 42500 is an organic compound with the formula [(H2NC6H4)3C]Cl. It is a magenta solid with a variety of uses as a dye. It is one of the four components of basic fuchsine. (The others are rosaniline, new fuchsine and magenta II.) It is structurally related to other triarylmethane dyes called methyl violets including crystal violet, which feature methyl groups on nitrogen.
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen (OBE/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with water (H2O). During the synthesis, ammonium chloride is also produced.

Acid fuchsin or fuchsine acid, (also called Acid Violet 19 and C.I. 42685) is an acidic magenta dye with the chemical formula C20H17N3Na2O9S3. It is a sodium sulfonate derivative of fuchsine. Acid fuchsin has wide use in histology, and is one of the dyes used in Masson's trichrome stain. This method is commonly used to stain cytoplasm and nuclei of tissue sections in the histology laboratory in order to distinguish muscle from collagen. The muscle stains red with the acid fuchsin, and the collagen is stained green or blue with Light Green SF yellowish or methyl blue. It can also be used to identify growing bacteria.
para-Dimethylaminobenzaldehyde is an organic compound containing amine and aldehyde moieties which is used in Ehrlich's reagent and Kovac's reagent to test for indoles. The carbonyl group typically reacts with the electron rich 2-position of the indole but may also react at the C-3 or N-1 positions. It may also be used for determination of hydrazine.
Verhoeff's stain, also known as Verhoeff's elastic stain (VEG) or Verhoeff–Van Gieson stain (VVG), is a staining protocol used in histology, developed by American ophthalmic surgeon and pathologist Frederick Herman Verhoeff (1874–1968) in 1908. The formulation is used to demonstrate normal or pathologic elastic fibers.