Related Research Articles

Glass is a non-crystalline solid that is often transparent, brittle and chemically inert. It has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics.

In optics, the refractive index of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.

Lead glass, commonly called crystal, is a variety of glass in which lead replaces the calcium content of a typical potash glass. Lead glass contains typically 18–40% lead(II) oxide (PbO), while modern lead crystal, historically also known as flint glass due to the original silica source, contains a minimum of 24% PbO. Lead glass is often desirable for a variety of uses due to its clarity. In marketing terms it is often called crystal glass.

John Bannister Goodenough was an American materials scientist, a solid-state physicist, and a Nobel laureate in chemistry. From 1996 he was a professor of Mechanical, Materials Science, and Electrical Engineering at the University of Texas at Austin. He is credited with identifying the Goodenough–Kanamori rules of the sign of the magnetic superexchange in materials, with developing materials for computer random-access memory and with inventing cathode materials for lithium-ion batteries.
Chalcogenide glass is a glass containing one or more chalcogens. Up until recently, chalcogenide glasses (ChGs) were believed to be predominantly covalently bonded materials and classified as covalent network solids. A most recent and extremely comprehensive university study of more than 265 different ChG elemental compositions, representing 40 different elemental families now shows that the vast majority of chalcogenide glasses are more accurately defined as being predominantly bonded by the weaker van der Waals forces of atomic physics and more accurately classified as van der Waals network solids. They are not exclusively bonded by these weaker vdW forces, and do exhibit varying percentages of covalency, based upon their specific chemical makeup. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties.
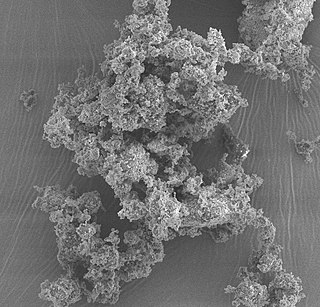
Bioactive glasses are a group of surface reactive glass-ceramic biomaterials and include the original bioactive glass, Bioglass. The biocompatibility and bioactivity of these glasses has led them to be used as implant devices in the human body to repair and replace diseased or damaged bones. Most bioactive glasses are silicate-based glasses that are degradable in body fluids and can act as a vehicle for delivering ions beneficial for healing. Bioactive glass is differentiated from other synthetic bone grafting biomaterials, in that it is the only one with anti-infective and angiogenic properties.

John Werner Cahn was an American scientist and recipient of the 1998 National Medal of Science. Born in Cologne, Weimar Germany, he was a professor in the department of metallurgy at the Massachusetts Institute of Technology (MIT) from 1964 to 1978. From 1977, he held a position at the National Institute of Standards and Technology. Cahn had a profound influence on the course of materials research during his career. One of the foremost authorities on thermodynamics, Cahn applied the basic laws of thermodynamics to describe and predict a wide range of physical phenomena.
Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.

The calculation of glass properties is used to predict glass properties of interest or glass behavior under certain conditions without experimental investigation, based on past data and experience, with the intention to save time, material, financial, and environmental resources, or to gain scientific insight. It was first practised at the end of the 19th century by A. Winkelmann and O. Schott. The combination of several glass models together with other relevant functions can be used for optimization and six sigma procedures. In the form of statistical analysis glass modeling can aid with accreditation of new data, experimental procedures, and measurement institutions.

Barium borate is an inorganic compound, a borate of barium with a chemical formula BaB2O4 or Ba(BO2)2. It is available as a hydrate or dehydrated form, as white powder or colorless crystals. The crystals exist in the high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.

The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.

Mikhail Mikhaylovich Shultz, was a Soviet/Russian physical chemist, and an artist.
Jack Gilman was a world-renowned material scientist in the field of mechanical properties of solids. In his lifetime he made major contributions to many areas of the field including dislocation behaviour of ceramics, disclination behaviour of polymers, and production of metal glasses.
William Houlder Zachariasen, more often known as W. H. Zachariasen, was a Norwegian-American physicist, specializing in X-ray crystallography and famous for his work on the structure of glass.

Mansoura University was founded in 1972 in Mansoura city, Egypt. It is in the middle of the Nile Delta. It is one of the biggest Egyptian universities and has contributed much to the cultural and scientific life in Mansoura and Egypt.

Helen Frances Gleeson OBE FInstP is a British physicist who specialises in soft matter and liquid crystals. She is Cavendish Professor and former Head of the School of Physics at the University of Leeds.

Lead bismuthate is a superconductor with the formula Pb(BiO3)2. has only been discovered in recent years in the laboratory as it is not naturally occurring. Lead bismuthate forms a pentavalent structure, significantly different from the regular ionic interactions of sodium bismuthate, but similar to that of strontium bismuthate. In the structure, six oxygen atoms are coordinated octahedrally to both the bismuth and lead atoms. The bismuth and oxygen atoms form negatively charged layers by creating repeating octahedral geometries. The positively charged lead atoms are then disbursed within the layers, forming a hexagonal unit cell, with a lead atom in each of the corners. The density of the crystal is 9.18 g/cm3. The formula weight is 233.99 g/mol. The volume of the crystal structure unit is 169.26 A3. Lattice parameters (a) is 5.321 angstroms.

Jacques Lucas is Professor Emeritus at the University of Rennes 1. Jacques Lucas is a solids-based chemist who specializes in the discovery of new lenses, contributing to their analysis, knowledge of their optical properties and their use in various fields. He is a member of the French Academy of sciences.
Ahmed Amin HamzaFAAS FRMS SPIE is an Egyptian emeritus professor of physics at the Faculty of Science, University of Mansoura. He was a former President and Vice President of the institution and an ex-President of the British University in Egypt. He is a member of African Academy of Sciences and a fellow of the institute of physics.
References
- ↑ "Hamdy Doweidar" . Retrieved 4 July 2022.
- ↑ "Mansoura University researchers obtain three patents" . Retrieved 4 July 2022.
- ↑ "Hamdy Doweidar" . Retrieved 4 July 2022.
- ↑ "Google Scholar" . Retrieved 4 July 2022.
- ↑ "50 years of dissertations at the University of Architecture and Civil Engineering and the Bauhaus University in Weimar" . Retrieved 3 July 2022.
- ↑ "Glass Research Laboratory" . Retrieved 4 July 2022.
- ↑ "Hamdy Doweidar" . Retrieved 4 July 2022.
- ↑ "Mansoura University scholars among the most influential in the world" . Retrieved 4 July 2022.