Related Research Articles

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth and or disassembly depending on the cell's requirements.

Smoothmuscle is one of the three major types of vertebrate muscle tissue, the others being skeletal and cardiac muscle. It can also be found in invertebrates and is controlled by the autonomic nervous system. It is non-striated, so-called because it has no sarcomeres and therefore no striations. It can be divided into two subgroups, single-unit and multi-unit smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium.

Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP-dependent pulling action in muscle contraction and pseudopod advancement. Microfilaments have a tough, flexible framework which helps the cell in movement.

Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm.
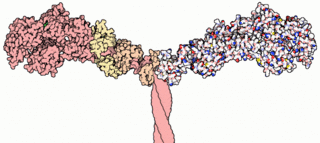
Myosins are a family of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility.
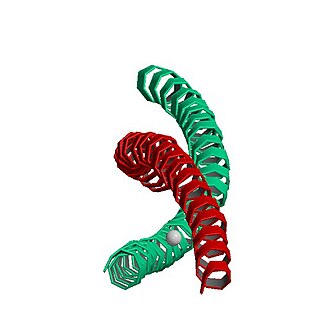
MYH7 is a gene encoding a myosin heavy chain beta (MHC-β) isoform expressed primarily in the heart, but also in skeletal muscles. This isoform is distinct from the fast isoform of cardiac myosin heavy chain, MYH6, referred to as MHC-α. MHC-β is the major protein comprising the thick filament that forms the sarcomeres in cardiac muscle and plays a major role in cardiac muscle contraction.
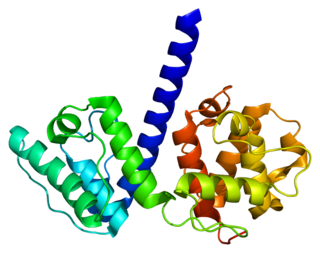
Plectin is a giant protein found in nearly all mammalian cells which acts as a link between the three main components of the cytoskeleton: actin microfilaments, microtubules and intermediate filaments. In addition, plectin links the cytoskeleton to junctions found in the plasma membrane that structurally connect different cells. By holding these different networks together, plectin plays an important role in maintaining the mechanical integrity and viscoelastic properties of tissues.

Nebulin is an actin-binding protein which is localized to the thin filament of the sarcomeres in skeletal muscle. Nebulin in humans is coded for by the gene NEB. It is a very large protein and binds as many as 200 actin monomers. Because its length is proportional to thin filament length, it is believed that nebulin acts as a thin filament "ruler" and regulates thin filament length during sarcomere assembly. Other functions of nebulin, such as a role in cell signaling, remain uncertain.
Actinin is a microfilament protein. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. Alpha-actinin is a part of the spectrin superfamily. This superfamily is made of spectrin, dystrophin, and their homologous and isoforms. In non-muscle cells, it is found by the actin filaments and at the adhesion sites.The lattice like arrangement provides stability to the muscle contractile apparatus. Specifically, it helps bind actin filaments to the cell membrane. There is a binding site at each end of the rod and with bundles of actin filaments.

Nebulette is a cardiac-specific isoform belonging to the nebulin family of proteins. It is encoded by the NEBL gene. This family is composed of 5 members: nebulette, nebulin, N-RAP, LASP-1 and LASP-2. Nebulette localizes to Z-discs of cardiac muscle and appears to regulate the length of actin thin filaments.
Meromyosin is a part of myosin. With regards to human anatomy myosin and actin constitute the basic functional unit of a muscle fiber, called sarcomere, playing a role in muscle contraction.

Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells.

Actin, cytoplasmic 2, or gamma-actin is a protein that in humans is encoded by the ACTG1 gene. Gamma-actin is widely expressed in cellular cytoskeletons of many tissues; in adult striated muscle cells, gamma-actin is localized to Z-discs and costamere structures, which are responsible for force transduction and transmission in muscle cells. Mutations in ACTG1 have been associated with nonsyndromic hearing loss and Baraitser-Winter syndrome, as well as susceptibility of adolescent patients to vincristine toxicity.

Myosin-9 also known as myosin, heavy chain 9, non-muscle or non-muscle myosin heavy chain IIa (NMMHC-IIA) is a protein which in humans is encoded by the MYH9 gene.

ACTC1 encodes cardiac muscle alpha actin. This isoform differs from the alpha actin that is expressed in skeletal muscle, ACTA1. Alpha cardiac actin is the major protein of the thin filament in cardiac sarcomeres, which are responsible for muscle contraction and generation of force to support the pump function of the heart.

Myosin heavy chain, α isoform (MHC-α) is a protein that in humans is encoded by the MYH6 gene. This isoform is distinct from the ventricular/slow myosin heavy chain isoform, MYH7, referred to as MHC-β. MHC-α isoform is expressed predominantly in human cardiac atria, exhibiting only minor expression in human cardiac ventricles. It is the major protein comprising the cardiac muscle thick filament, and functions in cardiac muscle contraction. Mutations in MYH6 have been associated with late-onset hypertrophic cardiomyopathy, atrial septal defects and sick sinus syndrome.

Rho-associated protein kinase or Rho-associated coiled-coil kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.

The myosin head is the part of the thick myofilament made up of myosin that acts in muscle contraction, by sliding over thin myofilaments of actin. Myosin is the major component of the thick filaments and most myosin molecules are composed of a head, neck, and tail domain; the myosin head binds to thin filamentous actin, and uses ATP hydrolysis to generate force and "walk" along the thin filament. Myosin exists as a hexamer of two heavy chains, two alkali light chains, and two regulatory light chains. The heavy chain can be subdivided into the globular head at the N-terminal and the coiled-coil rod-like tail at the C-terminal, although some forms have a globular region in their C-terminal.
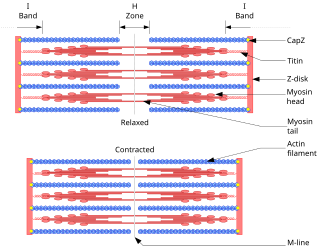
The sliding filament theory explains the mechanism of muscle contraction based on muscle proteins that slide past each other to generate movement. According to the sliding filament theory, the myosin of muscle fibers slide past the actin during muscle contraction, while the two groups of filaments remain at relatively constant length.

Calponin 1 is a basic smooth muscle protein that in humans is encoded by the CNN1 gene.
References
- ↑ Sellers, J. R. (1985). "Mechanism of the phosphorylation-dependent regulation of smooth muscle heavy meromyosin". The Journal of Biological Chemistry. 260 (29): 15815–15819. doi: 10.1016/S0021-9258(17)36331-7 . PMID 2933403.
- ↑ Woodrum, D. T.; Rich, S. A.; Pollard, T. D. (1975). "Evidence for biased bidirectional polymerization of actin filaments using heavy meromyosin prepared by an improved method". Journal of Cell Biology. 67 (1): 231–237. doi:10.1083/jcb.67.1.231. PMC 2109590 . PMID 240859.