
The noble gases make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six naturally occurring noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).
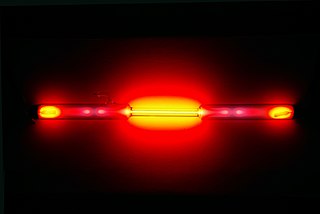
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, νέον, neuter singular form of νέος, meaning 'new'. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates.

The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers. The table is divided into four roughly rectangular areas called blocks. The rows of the table are called periods, and the columns are called groups. Elements from the same group of the periodic table show similar chemical characteristics. Trends run through the periodic table, with nonmetallic character increasing from left to right across a period, and from down to up across a group, and metallic character increasing in the opposite direction. The underlying reason for these trends is electron configurations of atoms. The periodic table exclusively lists electrically neutral atoms that have an equal number of positively charged protons and negatively charged electrons and puts isotopes at the same place. Other atoms, like nuclides and isotopes, are graphically collected in other tables like the tables of nuclides.

In chemistry, a transition metal is a chemical element in the d-block of the periodic table, though the elements of group 12 are sometimes excluded. The lanthanide and actinide elements are called inner transition metals and are sometimes considered to be transition metals as well.

In physics and chemistry, ionization energy (IE) (American English spelling), ionisation energy (British English spelling) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as

In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively.

In chemistry, a group is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the 14 f-block columns, between groups 2 and 3, are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms, because most chemical properties are dominated by the orbital location of the outermost electron.

A period on the periodic table is a row of chemical elements. All elements in a row have the same number of electron shells. Each next element in a period has one more proton and is less metallic than its predecessor. Arranged this way, elements in the same group (column) have similar chemical and physical properties, reflecting the periodic law. For example, the halogens lie in the second-to-last group and share similar properties, such as high reactivity and the tendency to gain one electron to arrive at a noble-gas electronic configuration. As of 2022, a total of 118 elements have been discovered and confirmed.
A period 1 element is one of the chemical elements in the first row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate periodic (recurring) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that analog elements fall into the same vertical columns. The first period contains fewer elements than any other row in the table, with only two: hydrogen and helium. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 1s orbital. Period 1 elements obey the duet rule in that they need two electrons to complete their valence shell.

In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron.

The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties. In the basic form, elements are presented in order of increasing atomic number, in the reading sequence. Then, rows and columns are created by starting new rows and inserting blank cells, so that rows (periods) and columns (groups) show elements with recurring properties. For example, all elements in group (column) 18 are noble gases that are largely—though not completely—unreactive.

Julius Lothar Meyer was a German chemist. He was one of the pioneers in developing the earliest versions of the periodic table of the chemical elements. The Russian chemist Dmitri Mendeleev and he had both worked with Robert Bunsen. Meyer never used his first given name and was known throughout his life simply as Lothar Meyer.
The atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Depending on the definition, the term may apply only to isolated atoms, or also to atoms in condensed matter, covalently bound in molecules, or in ionized and excited states; and its value may be obtained through experimental measurements, or computed from theoretical models. Under some definitions, the value of the radius may depend on the atom's state and context.

Alternative periodic tables are tabulations of chemical elements differing in their organization from the traditional depiction of the periodic system.
Charge number (z) refers to a quantized value of electric charge, with the quantum of electric charge being the elementary charge, so that the charge number equals the electric charge (q) in coulombs divided by the elementary-charge constant (e), or z = q/e. The charge numbers for ions (and also subatomic particles) are written in superscript, e.g. Na+ is a sodium ion with charge number positive one (an electric charge of one elementary charge). Atomic numbers (Z) are a special case of charge numbers, referring to the charge number of an atomic nucleus, as opposed to the net charge of an atom or ion. All particles of ordinary matter have integer-value charge numbers, with the exception of quarks, which cannot exist in isolation under ordinary circumstances (the strong force keeps them bound into hadrons of integer charge numbers).
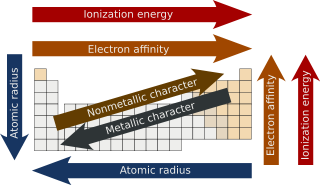
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. They were discovered by the Russian chemist Dmitri Mendeleev in the year 1863. Major periodic trends include atomic radius, ionization energy, electron affinity, electronegativity, valency and metallic character. These trends exist because of the similar electronic configuration of the elements within their respective groups or periods and because of the periodic nature of the elements. These give a qualitative assessment of the properties of each element.
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond to the principal quantum numbers (n = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, ...). A useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements represents an electron shell.

The Periodic Table: Elements with Style is a 2007 children's science book created by Simon Basher and written by Adrian Dingle. It is the first book in Basher's science series, which includes Physics: Why Matter Matters!, Biology: Life As We Know It, Astronomy: Out of this World!, Rocks and Minerals: A Gem of a Book, and Planet Earth: What Planet Are You On?, each of which is 128 pages long.
Periodic systems of molecules are charts of molecules similar to the periodic table of the elements. Construction of such charts was initiated in the early 20th century and is still ongoing.

Veli Pekka Pyykkö is a Finnish academic. He was professor of Chemistry at the University of Helsinki. From 2009–2012, he was the chairman of the International Academy of Quantum Molecular Science. He is known for his extension to the periodic table of elements, known as the Pyykkö model.















