Related Research Articles

Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells, in turn relating greatly to the understanding of tissues and organs as well as organism structure and function. Biochemistry is closely related to molecular biology, the study of the molecular mechanisms of biological phenomena.

Chymotrypsin (EC 3.4.21.1, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid N-terminal to the scissile amide bond (the P1 position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S1 position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P1 side chain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine at the P1 position.

Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.

Tryptophan synthase or tryptophan synthetase is an enzyme that catalyses the final two steps in the biosynthesis of tryptophan. It is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae. However, it is absent from Animalia. It is typically found as an α2β2 tetramer. The α subunits catalyze the reversible formation of indole and glyceraldehyde-3-phosphate (G3P) from indole-3-glycerol phosphate (IGP). The β subunits catalyze the irreversible condensation of indole and serine to form tryptophan in a pyridoxal phosphate (PLP) dependent reaction. Each α active site is connected to a β active site by a 25 angstrom long hydrophobic channel contained within the enzyme. This facilitates the diffusion of indole formed at α active sites directly to β active sites in a process known as substrate channeling. The active sites of tryptophan synthase are allosterically coupled.

A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst. Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound.

Sir Frederick Gowland Hopkins was an English biochemist who was awarded the Nobel Prize in Physiology or Medicine in 1929, with Christiaan Eijkman, for the discovery of vitamins. He also discovered the amino acid tryptophan, in 1901. He was President of the Royal Society from 1930 to 1935.
The Bradford protein assay was developed by Marion M. Bradford in 1976. It is a quick and accurate spectroscopic analytical procedure used to measure the concentration of protein in a solution. The reaction is dependent on the amino acid composition of the measured proteins.
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.

Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
The Lowry protein assay is a biochemical assay for determining the total level of protein in a solution. The total protein concentration is exhibited by a color change of the sample solution in proportion to protein concentration, which can then be measured using colorimetric techniques. It is named for the biochemist Oliver H. Lowry who developed the reagent in the 1940s. His 1951 paper describing the technique is the most-highly cited paper ever in the scientific literature, cited over 300,000 times.
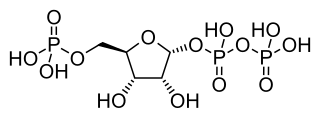
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase:

Tryptophan hydroxylase (TPH) is an enzyme (EC 1.14.16.4) involved in the synthesis of the monoamine neurotransmitter serotonin. Tyrosine hydroxylase, phenylalanine hydroxylase, and tryptophan hydroxylase together constitute the family of biopterin-dependent aromatic amino acid hydroxylases. TPH catalyzes the following chemical reaction

Dorothy Mary Moyle Needham FRS was an English biochemist known for her work on the biochemistry of muscle. She was married to biochemist Joseph Needham.
The Adamkiewicz reaction is part of a biochemical test used to detect the presence of the amino acid tryptophan in proteins. When concentrated sulfuric acid is combined with a solution of protein and glyoxylic acid, a red/purple colour is produced. It was named after its discoverer, Albert Wojciech Adamkiewicz. Pure sulfuric acid and a minimal amount of pure formaldehyde, along with an oxidizing agent introduced into the sulfuric acid, allow the reaction to proceed.

The xanthoproteic reaction is a method that can be used to detect a presence of protein soluble in a solution, using concentrated nitric acid. The test gives a positive result in amino acids carrying aromatic groups, especially in the presence of tyrosine. If the test is positive the proof is neutralized with an alkali, turning dark yellow. The yellow colour is due to xanthoproteic acid which is formed due to nitration of certain amino acids, most common examples being tyrosine and tryptophan. This chemical reaction is a qualitative test, determining the presence or absence of proteins.
The Acree-Rosenheim reaction is a chemical test used for detecting the presence of tryptophan in proteins. A protein mixture is mixed with formaldehyde. Concentrated sulfuric acid is added to form two layers. A purple ring appears between the two layers if the test is positive for tryptophan.
The Pauly reaction is a chemical test used for detecting the presence of tyrosine or histidine in proteins. It is named after German chemist Hermann Pauly, who first described the reaction. When proteins containing either tyrosine or histidine are reacted with diazotized sulfanilic acid under alkaline conditions, a red color is formed by a coupling reaction.
Heller's test is a chemical test that shows that strong acids cause the denaturation of precipitated proteins. Concentrated nitric acid is added to a protein solution from the side of the test tube to form two layers. A white ring appears between the two layers if the test is positive. Heller's test is commonly used to test for the presence of proteins in urine. This test was discovered by the Austrian Chemist, Johann Florian Heller (1813-1871).
Salkowski's test, also known simply as Salkowski test, is a qualitative chemical test, that is used in chemistry and biochemistry for detecting a presence of cholesterol and other sterols. This biochemical method got its name after German biochemist Ernst Leopold Salkowski, who is known for development of multiple new chemical tests, that are used for detection of different kinds of molecules. A solution that has tested positive on the Salkowski's test becomes red and gets yellow glow.
References
- ↑ R.A. Joshi (2006). Question Bank of Biochemistry. New Age International. p. 64. ISBN 978-81-224-1736-4.
- ↑ Debajyoti Das (1980). Biochemistry. Academic Publishers. p. 56. ISBN 978-93-80599-17-5.
- ↑ P. M. Swamy (2008). Laboratory Manual on Biotechnology. Rastogi Publications. p. 90. ISBN 978-81-7133-918-1.
- ↑ Chatterjea (1 January 2004). Textbook of Biochemistry for Dental/Nursing/Pharmacy Students. Jaypee Brothers Publishers. p. 51. ISBN 978-81-8061-204-6.
- ↑ Hopkins, Frederick Gowland; Cole, Sydney W. (1901). "On the proteid reaction of Adamkiewicz, with contributions to the chemistry of glyoxylic acid". Proceedings of the Royal Society. 68 (442–450): 21–33. doi:10.1098/rspl.1901.0008. S2CID 84899015.