
Pentafluoroethyl iodide is a suggested component of a fire-extinguishing composition. It is a very dense gas.
A cyanogen halide is a molecule consisting of cyanide and a halogen. Cyanogen halides are chemically classified as pseudohalogens.

Dibromofluoromethane is a mixed halomethane. It is soluble in alcohol, acetone, benzene and chloroform. It is prepared from dibromomethane and antimony(III) fluoride.
Heinrich Hubert Maria Josef Houben was a German chemist. He made achievements within ketone synthesis, terpenes, and camphor studies. After being wounded several times on the front lines in World War I, Houben was made head of the war laboratory. He improved the Hoesch reaction which is now normally called Houben-Hoesch reaction. Houben organized and made a major rework of the book Methods of Organic Chemistry which is now referred to as Houben-Weyl Methods of Organic Chemistry.

[1.1.1]Propellane is an organic compound, the simplest member of the propellane family. It is a hydrocarbon with formula C5H6 or C2(CH2)3. The molecular structure consists of three rings of three carbon atoms each, sharing one C–C bond.

[2.2.2]Propellane, formally tricyclo[2.2.2.01,4]octane is an organic compound, a member of the propellane family. It is a hydrocarbon with formula C8H12, or C2(C2H4)3. Its molecule has three rings with four carbon atoms each, sharing one C–C bond.
Dimethylmagnesium is an organomagnesium compound. It is a white pyrophoric solid. Dimethylmagnesium is used in the synthesis of organometallic compounds.

Ullmann's Encyclopedia of Industrial Chemistry is a major reference work related to industrial chemistry by chemist Fritz Ullmann, first published in 1914, and exclusively in German as "Enzyklopädie der Technischen Chemie" until 1984.
Theodor Weyl was a German chemist and hygienist born in Berlin.
The International Symposium on Fluorine Chemistry (ISFC) is an academic conference where researchers present their most recent original results on the chemistry of fluorine and its derivatives. Communications deal with all compounds of fluorine, from hydrogen fluoride to fluoropolymers and other fluorocarbons. Participants to editions from 18th ISFC onwards have been nearly 500, most of them were from academia, researchers from fluorochemical industry being the second largest group.
Penicillium tardum is an anamorph species of fungus in the genus Penicillium which produces rugulosin.

Calicene or triapentafulvalene is a hydrocarbon of the fulvalene class with chemical formula C8H6, composed of a cyclopentadiene ring and a cyclopropene ring linked by a double bond. Its name is derived from the Latin calix meaning "goblet", from its shape.
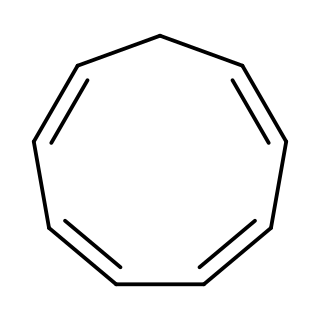
Cyclononatetraene is an organic compound with the formula C9H10. It was first prepared in 1969 by protonation of the corresponding aromatic anion (described below). It is unstable and isomerizes with a half-life of 50 minutes at room temperature to 3a,7a-dihydro-1H-indene via a thermal 6π disrotatory electrocyclic ring closing. Upon exposure to ultraviolet light, it undergoes a photochemical 8π electrocyclic ring closing to give bicyclo[6.1.0]nona-2,4,6-triene.

Samarium(II) bromide is an inorganic compound with the chemical formula SmBr
2. It is a brown solid that is insoluble in most solvents but degrades readily in air.
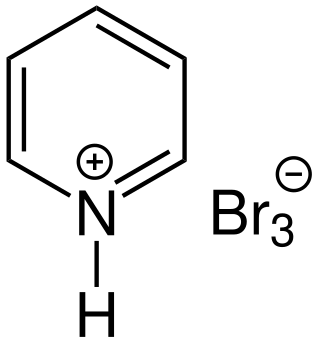
Pyridinium perbromide (also called pyridinium bromide perbromide, pyridine hydrobromide perbromide, or pyridinium tribromide) is an organic chemical composed of a pyridinium cation and a tribromide anion. It can also be considered as a complex containing pyridinium bromide—the salt of pyridine and hydrogen bromide—with an added bromine (Br2). The chemical is a solid whose reactivity is similar to that of bromine. It is thus a strong oxidizing agent used as a source of electrophilic bromine in halogenation reactions. The analogous quinoline compound behaves similarly.

Cyanogen azide is a chemical compound with the chemical formula CN4, or more precisely −N=N+=N−C≡N. It is an azide compound of carbon and nitrogen. It is an oily, colourless liquid at room temperature. It is a highly explosive chemical that is soluble in most organic solvents, and normally handled in dilute solution in this form. It was first synthesised by F. D. Marsh at DuPont in the early 1960s. There had been earlier claims of discovering it as a crystalline solid, which were incorrect.
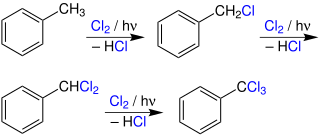
Photochlorination is a chlorination reaction that is initiated by light. Usually a C-H bond is converted to a C-Cl bond. Photochlorination is carried out on an industrial scale. The process is exothermic and proceeds as a chain reaction initiated by the homolytic cleavage of molecular chlorine into chlorine radicals by ultraviolet radiation. Many chlorinated solvents are produced in this way.

Cyanuric bromide is a heterocyclic compound with formula C3N3Br3. It contains a six-membered ring of alternating nitrogen and carbon atoms, with a bromine atom attached to each carbon. It is formed by the spontaneous trimerisation of cyanogen bromide.

1-Fluorohexane is a chemical compound from the group of aliphatic saturated halogenated hydrocarbons. The chemical formula is CH3(CH2)5F.












