Related Research Articles

The halogens are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). The artificially created element 117, tennessine (Ts), may also be a halogen. In the modern IUPAC nomenclature, this group is known as group 17.

Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a measure of the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity.

Methoxides are organic salts and the simplest alkoxides. Sodium methoxide and potassium methoxide have widespread use, though other metal-cation variants such as lithium methoxide, rubidium methoxide, and caesium methoxide exist as well.

Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the chemical formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant.

Hydroiodic acid is an aqueous solution of hydrogen iodide (HI). It is a strong acid, one that is ionized completely in an aqueous solution. It is colorless. Concentrated solutions are usually 48% to 57% HI.
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light and air because it forms peroxides.

Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) with a fluorine atom substituted for one of the hydrogen atoms. It is used in semiconductor manufacturing processes as an etching gas in plasma etch reactors.

Hydrogen iodide is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.
Cyanogen iodide or iodine cyanide (ICN) is a pseudohalogen composed of iodine and the cyanide group. It is a relatively volatile and highly toxic inorganic compound. It occurs as white crystals that react slowly with water to form hydrogen cyanide.

Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt.
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.

Cadmium fluoride (CdF2) is a mostly water-insoluble source of cadmium used in oxygen-sensitive applications, such as the production of metallic alloys. In extremely low concentrations (ppm), this and other fluoride compounds are used in limited medical treatment protocols. Fluoride compounds also have significant uses in synthetic organic chemistry. The standard enthalpy has been found to be -167.39 kcal. mole−1 and the Gibbs energy of formation has been found to be -155.4 kcal. mole−1, and the heat of sublimation was determined to be 76 kcal. mole−1.

Propan-1-ol is a primary alcohol with the formula CH3CH2CH2OH and sometimes represented as PrOH or n-PrOH. It is a colorless liquid and an isomer of 2-propanol. It is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent.
International Chemical Safety Cards (ICSC) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace and the main target users are therefore workers and those responsible for occupational safety and health. The ICSC project is a joint venture between the World Health Organization (WHO) and the International Labour Organization (ILO) with the cooperation of the European Commission (EC). This project began during the 1980s with the objective of developing a product to disseminate the appropriate hazard information on chemicals at the workplace in an understandable and precise way.
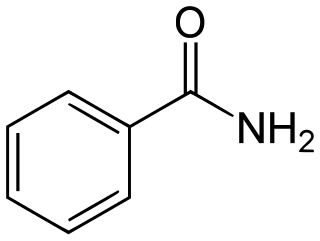
Benzamide is a white solid with the chemical formula of C6H5C(O)NH2. It is the simplest amide derivative of benzoic acid. It is slightly soluble in water, and soluble in many organic solvents. A number of substituted benzamides are commercial drugs: sulpiride, remoxipride, amisulpride, tiapride, sultopride, veralipride, aminohippuric acid, cisapride, imatinib, and procainamide.

Vinyl fluoride is an organic halide with the chemical formula C2H3F. It is a colorless gas with a faint etherlike odor. It is used as the monomeric precursor to the fluoropolymer polyvinylfluoride.

Magnesium iodide is the name for the chemical compounds with the formulas MgI2 and its various hydrates MgI2(H2O)x. These salts are typical ionic halides, being highly soluble in water.

Beryllium iodide is the inorganic compound with the formula BeI2. It is a hygroscopic white solid.
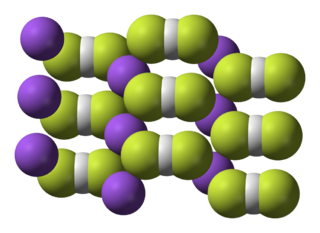
Sodium bifluoride is the inorganic compound with the formula NaHF2. It is a salt of sodium cation (Na+) and bifluoride anion (HF2−). It is a white, water-soluble solid that decomposes upon heating. Sodium bifluoride is non-flammable, hygroscopic, and has a pungent smell. Sodium bifluoride has a number of applications in industry.