Related Research Articles

In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (cations) and negatively charged ions (anions), which results in a compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry. It is also known as red iron oxide, especially when used in pigments.

In colloidal chemistry, flocculation is a process by which colloidal particles come out of suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion and are not truly dissolved in solution.
Fenton's reagent is a solution of hydrogen peroxide (H2O2) and an iron catalyst (typically iron(II) sulfate, FeSO4). It is used to oxidize contaminants or waste water as part of an advanced oxidation process. Fenton's reagent can be used to destroy organic compounds such as trichloroethylene and tetrachloroethylene (perchloroethylene). It was developed in the 1890s by Henry John Horstman Fenton as an analytical reagent.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.
Inner sphere complex is a type of surface complex that refers to the surface chemistry changing a water-surface interface to one without water molecules bridging a ligand to the metal ion. Formation of inner sphere complexes occurs when ions bind directly to the surface with no intervening water molecules. These types of surface complexes are restricted to ions that have a high affinity for surface sites and include specifically adsorbed ions that can bind to the surface through covalent bonding.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
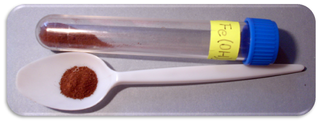
Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).
Soil chemistry is the study of the chemical characteristics of soil. Soil chemistry is affected by mineral composition, organic matter and environmental factors. In the early 1870s a consulting chemist to the Royal Agricultural Society in England, named J. Thomas Way, performed many experiments on how soils exchange ions, and is considered the father of soil chemistry. Other scientists who contributed to this branch of ecology include Edmund Ruffin, and Linus Pauling.

Sodium persulfate is the inorganic compound with the formula Na2S2O8. It is the sodium salt of peroxydisulfuric acid, H2S2O8, an oxidizing agent. It is a white solid that dissolves in water. It is almost non-hygroscopic and has good shelf-life.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.
Illuvium is material displaced across a soil profile, from one layer to another one, by the action of rainwater. The removal of material from a soil layer is called eluviation. The transport of the material may be either mechanical or chemical. The process of deposition of illuvium is termed illuviation. It is a water-assisted transport in a basically vertical direction, as compared to alluviation, the horizontal running water transfer. The resulting deposits are called illuvial deposits. Cutans are a type of illuvial deposit.

Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hydrothermal hot springs and scales, soils, and areas affected by mining. It can be precipitated directly from oxygenated iron-rich aqueous solutions, or by bacteria either as a result of a metabolic activity or passive sorption of dissolved iron followed by nucleation reactions. Ferrihydrite also occurs in the core of the ferritin protein from many living organisms, for the purpose of intra-cellular iron storage.
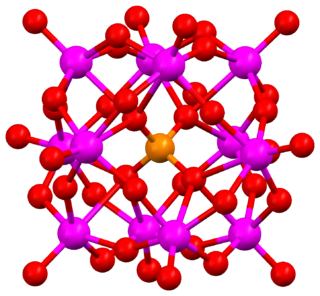
The Keggin structure is the best known structural form for heteropoly acids. It is the structural form of α-Keggin anions, which have a general formula of [XM12O40]n−, where X is the heteroatom, M is the addendum atom, and O represents oxygen. The structure self-assembles in acidic aqueous solution and is a commonly used type of polyoxometalate catalysts.

Laterite is a soil type rich in iron and aluminium and is commonly considered to have formed in hot and wet tropical areas. Nearly all laterites are of rusty-red coloration, because of high iron oxide content. They develop by intensive and prolonged weathering of the underlying parent rock, usually when there are conditions of high temperatures and heavy rainfall with alternate wet and dry periods. The process of formation is called laterization. Tropical weathering is a prolonged process of chemical weathering which produces a wide variety in the thickness, grade, chemistry and ore mineralogy of the resulting soils. The majority of the land area containing laterites is between the tropics of Cancer and Capricorn.
Germanium(II) hydroxide, normally written as Ge(OH)2, is a poorly characterised compound, sometimes called hydrous germanium(II) oxide or germanous hydroxide. It was first reported by Winkler in 1886.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Photogeochemistry merges photochemistry and geochemistry into the study of light-induced chemical reactions that occur or may occur among natural components of Earth's surface. The first comprehensive review on the subject was published in 2017 by the chemist and soil scientist Timothy A Doane, but the term photogeochemistry appeared a few years earlier as a keyword in studies that described the role of light-induced mineral transformations in shaping the biogeochemistry of Earth; this indeed describes the core of photogeochemical study, although other facets may be admitted into the definition.
Steven L. Suib is an American inorganic chemist, academic and researcher. He is a Board of Trustees Distinguished Professor of Chemistry at University of Connecticut. He is a director of the Institute of Materials Science and of the Center for Advanced Microscopy and Materials Analysis.
References
- ↑ Weiser, H. B. (1920). "Hydrous Oxides. I". The Journal of Physical Chemistry. 24 (4): 277–328. doi:10.1021/j150202a003.
- ↑ Weiser, H. B. (1923). "Hydrous Oxides. V". The Journal of Physical Chemistry. 27 (6): 501–532. doi:10.1021/j150231a001.
- ↑ Heitner-Wirguin, C.; Albu-Yaron, A. (1966). "Hydrous oxides and their cation exchange properties—II Structure and equilibrium experiments". Journal of Inorganic and Nuclear Chemistry. 28 (10): 2379–2384. doi:10.1016/0022-1902(66)80129-X.
- ↑ Karthikeyan, K.G; Elliott, Herschel A. (1999). "Surface Complexation Modeling of Copper Sorption by Hydrous Oxides of Iron and Aluminum". Journal of Colloid and Interface Science. 220 (1): 88–95. Bibcode:1999JCIS..220...88K. doi:10.1006/jcis.1999.6507. PMID 10550244.
- ↑ Benjamin, Mark M.; Lawler, Desmond F. (13 June 2013). Water Quality Engineering: Physical / Chemical Treatment Processes. ISBN 9781118632277.
- ↑ Paunovic, Milan; Scherson, Daniel (1997). Proceedings of the Third Symposium on Electrochemically Deposited Thin Films. ISBN 9781566771696.
- ↑ Brandon, Erik J. (2003). Micropower and Microdevices: Proceedings of the International Symposium. ISBN 9781566773874.
- ↑ Rycroft, David W.; Amer, M. H. (1995). Prospects for the Drainage of Clay Soils. ISBN 9789251036242.