
Imidazole alkaloids are a group of alkaloids whose basic structure contains the imidazole ring system. [1]

Imidazole alkaloids are a group of alkaloids whose basic structure contains the imidazole ring system. [1]


In nature, imidazole alkaloids are found both as secondary plant compounds and as byproducts of histidine metabolism in marine organisms. One well-known imidazole alkaloid is pilocarpine, which is present in the leaves of Paraguay jaborandi.
For instance, from the seeds of garden cress (Lepidium sativum), various monomeric imidazole alkaloids can be isolated, including semilepidinoside A and semilepidinoside B, as well as dimeric forms such as lepidins B, C, D, E, and F. [2] In the sponge Leucetta chagosensis , a variety of imidazole alkaloids, such as 2-deoxy-2-aminokealiiquinone and Naamine C, have been identified. [3] Imbricatin has been isolated from the starfish Dermasterias imbricata , while martensin A has been obtained from the red alga Martensia fragilis . Notably, the marine sponge Pseudaxinyssa contains odilin. [1]
The Maca plant contains relevant amounts of lepidilin.
A well-known imidazole alkaloid is pilocarpine. Other representatives include. cynodin, cynometrin and odilin. [1]

Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. More rarely still, they may contain elements such as phosphorus, chlorine, and bromine.

Myrosinase is a family of enzymes involved in plant defense against herbivores, specifically the mustard oil bomb. The three-dimensional structure has been elucidated and is available in the PDB.

5-Bromo-DMT (5-bromo-N,N-dimethyltryptamine) is a psychedelic brominated indole alkaloid found in the sponges Smenospongia aurea and Smenospongia echina, as well as in Verongula rigida alongside 5,6-Dibromo-DMT and seven other alkaloids. It is the 5-bromo derivative of DMT, a psychedelic found in many plants and animals.

Toxiferine is a curare toxin. It is a bisindole alkaloid derived from Strychnos toxifera and a nicotinic acetylcholine receptor antagonist. This alkaloid is the main toxic component of Calabash curare, and one of the most toxic plant alkaloids known. The lethal dose (LD50) for mice has been determined as 10 - 60 µg/kg by intravenous administration. It is a muscle relaxant that causes paralysis of skeletal muscle, which takes approximately 2 hours to recovery for a moderate dose, and 8 hours of total paralysis with a 20-fold paralytic dose. The paralysis can be antagonized by neostigmine

Palau'amine is a toxic alkaloid compound synthesized naturally by certain species of sea sponges. The name of the molecule derives from the island nation of Palau, near where the first sponge species discovered to produce it, Stylotella agminata, is found. It has since been isolated in other sponges, including Stylissa massa.

Steroidal alkaloids have the basic steroidal skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic cyclopentanoperhydrophenanthrene skeleton that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in Chonemorpha fragrans, 'chonemorphine' was used to treat intestinal infections in Wistar rats..

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Gigactonine is a naturally occurring diterpene alkaloid first isolated from Aconitum gigas. It occurs widely in the Ranunculaceae plant family. The polycyclic ring system of this chemical compound contains nineteen carbon atoms and one nitrogen atom, which is the same as in aconitine and this is reflected in its preferred IUPAC name.

Oroidin is a bromopyrrole alkaloid, originally isolated from marine sponges in the genus Agelas. Its complex structure leads to wide biological activities, which makes Oroidin a potential drug candidate for various diseases. It also serves as chemical defense in marine sponges.

Plakoridine A is an alkaloid isolated from the marine sponge Plakortis sp. There are three plakoridines known, named plakoridine A, B, and C.

Leucetta is a genus of sponges in the family Leucettidae, which was first described in 1872 by Ernst Haeckel. The type species is Leucetta primigenia Haeckel, 1872 by subsequent designation.
Erythrina alkaloids, generally containing benzyl-tetrahydroisoquinoline structure, are widely distributed in Erythrina species, a genus of plants which belong to the Fabaceae family in tropical and subtropical regions. The Erythrina alkaloids can be found in several organs of Erythrina trees but are primarily found in their seeds. They display several unique properties, and are the subject of active scientific research relating to their synthesis and bioactivity.

Pseudoceratina is a genus of sponge within the family Pseudoceratinidae. They are characterized by possession of a dendritic fiber skeleton lacking laminar bark but containing pith. They have been found in a variety of habitats including the Great Barrier reef, the Red Sea, and Jamaica. Sponges of this genus have a microbiome known to produce a variety of chemicals that are used in pharmaceutical and anti-fouling activities. Notably, a species in this genus produces a chemical that is effective in inhibiting the migration of metastatic breast cancer cells.
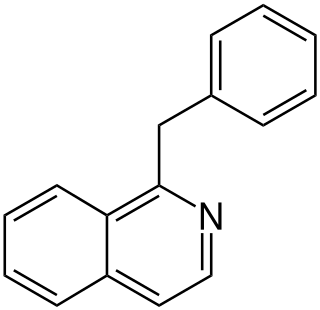
The benzylisoquinoline alkaloids are natural products that can be classified as isoquinoline alkaloids and are derived from benzylisoquinoline. They also include the benzyl(tetrahydro)isoquinoline alkaloids.
Bisbenzylisoquinoline alkaloids are natural products found primarily in the plant families of the barberry family, the Menispermaceae, the Monimiaceae, and the buttercup family.

Indolizidine alkaloids are natural products from various alkaloid groups whose structure can be derived from indolizidine.

Corydalis Alkaloids are categorized as natural products of the isoquinoline alkaloid type.

The pyrrolidine alkaloids are natural products chemically derived from pyrrolidine.

Areca alkaloids are a group of piperidine alkaloids found in the areca nut, the seeds of the areca palm.
Apocynaceae alkaloids are natural products found in the plant family of the dogbane family (Apocynaceae).