Aromatic compounds are those chemical compounds that contain one or more rings with pi electrons delocalized all the way around them. In contrast to compounds that exhibit aromaticity, aliphatic compounds lack this delocalization. The term "aromatic" was assigned before the physical mechanism determining aromaticity was discovered, and referred simply to the fact that many such compounds have a sweet or pleasant odour; however, not all aromatic compounds have a sweet odour, and not all compounds with a sweet odour are aromatic. Aromatic hydrocarbons, or arenes, are aromatic organic compounds containing solely carbon and hydrogen atoms. The configuration of six carbon atoms in aromatic compounds is called a "benzene ring", after the simple aromatic compound benzene, or a phenyl group when part of a larger compound.
Pyrimidine is an aromatic heterocyclic organic compound similar to pyridine. One of the three diazines, it has the nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine. In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U).
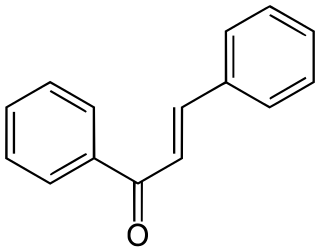
Chalcone is an aromatic ketone and an enone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones or chalconoids. Alternative names for chalcone include benzylideneacetophenone, phenyl styryl ketone, benzalacetophenone, β-phenylacrylophenone, γ-oxo-α,γ-diphenyl-α-propylene, and α-phenyl-β-benzoylethylene.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.

Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.

The Biginelli reaction is a multiple-component chemical reaction that creates 3,4-dihydropyrimidin-2(1H)-ones 4 from ethyl acetoacetate 1, an aryl aldehyde, and urea 3. It is named for the Italian chemist Pietro Biginelli.

Isatin, also known as tribulin, is an organic compound derived from indole with formula C8H5NO2. The compound was first obtained by Otto Linné Erdman and Auguste Laurent in 1840 as a product from the oxidation of indigo dye by nitric acid and chromic acids.
The Wurtz–Fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. This modification of the Wurtz reaction is considered a separate process and is named for both scientists.

The ANRORC mechanism in organic chemistry describes a special type of substitution reaction. ANRORC stands for Addition of the Nucleophile, Ring Opening, and Ring Closure in nucleophilic attack on ring systems and it helps to explain product formation and distribution in some nucleophilic substitutions especially in heterocyclic compounds. It is widely used in the medicinal chemistry.
The Cook–Heilbron thiazole synthesis highlights the formation of 5-aminothiazoles through the chemical reaction of α-aminonitriles or aminocynoacetates with dithioacids, carbon disulphide, carbon oxysulfide, or isothiocynates at room temperature and under mild or aqueous conditions. Variation of substituents at the 2nd and 4th position of the thiazole is introduced by selecting different combinations of starting reagents.

Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp2-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of aromatic organosulfur compounds. A few 1,2-dithietes have been isolated.
Organobismuth chemistry is the chemistry of organometallic compounds containing a carbon to bismuth chemical bond. According to one reviewer, applications are rare even though bismuth and bismuth compounds are the least toxic among the heavy metals and are relatively cheap. The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi. The first reported use of bismuth in organic chemistry was in oxidation of alcohols by Challenger in 1934 (using Ph3Bi(OH)2). Knowledge about methylated species of bismuth in environmental and biological media is very limited. Organobismuth heterocycles are bismole and bismabenzene. For reviews, see the cited articles
Thiete is a heterocyclic compound containing an unsaturated four-membered ring with three carbon atoms and one sulfur atom. It is more commonly encountered not on its own, but in anellated derivatives, several of which have been synthesized. Thietes are generally not very stable.
The Minisci reaction is a named reaction in organic chemistry. It is a nucleophilic radical substitution to an electron deficient aromatic compound, most commonly the introduction of an alkyl group to a nitrogen containing heterocycle. The reaction was published about in 1971 by F. Minisci. In the case of N-Heterocycles, the conditions must be acidic to ensure protonation of said heterocycle. A typical reaction is that between pyridine and pivalic acid with silver nitrate, sulfuric acid and ammonium persulfate to form 2-tert-butylpyridine. The reaction resembles Friedel-Crafts alkylation but with opposite reactivity and selectivity.

AM-1220 is a drug that acts as a potent and moderately selective agonist for the cannabinoid receptor CB1, with around 19 times selectivity for CB1 over the related CB2 receptor. It was originally invented in the early 1990s by a team led by Thomas D'Ambra at Sterling Winthrop, but has subsequently been researched by many others, most notably the team led by Alexandros Makriyannis at the University of Connecticut. The (piperidin-2-yl)methyl side chain of AM-1220 contains a stereocenter, so there are two enantiomers with quite different potency, the (R)-enantiomer having a Ki of 0.27 nM at CB1 while the (S)-enantiomer has a much weaker Ki of 217 nM.
Arthur William Pryor was an Australian physicist known for his contributions to neutron diffraction and infrared laser isotope separation. Pryor authored and co-authored a number of papers in the field of crystallography and he also co-authored, with B. T. M. Willis, the book Thermal Vibrations in Crystallography.

Werner Urland is a German chemist whose name is imprinted in the pioneering implementation of the Angular Overlap Model for the interpretation of optical and magnetic properties of rare-earth coordination compounds. This approach receives a renewed value in the context of the vogue around the lanthanide-based new materials, such as achieving magnets at molecular scale, or designing new phosphor materials.

Imidazothiazoles are a class of chemical compounds containing a bicyclic heterocycle consisting of an imidazole ring fused to a thiazole ring. The structure contains three heteroatoms: two nitrogen atoms and one sulfur atom. Imidazothiazole derivatives show a broad spectrum of physiological activity such as anticancer, antipsychotic, antimicrobial, antifungal, and anthelmintic.

The Mizoroki−Heck coupling of aryl halides and alkenes to form C(sp2)–C(sp2) bonds has become a staple transformation in organic synthesis, owing to its broad functional group compatibility and varied scope. In stark contrast, the palladium-catalyzed reductive Heck reaction has received considerably less attention, despite the fact that early reports of this reaction date back almost half a century. From the perspective of retrosynthetic logic, this transformation is highly enabling because it can forge alkyl–aryl linkages from widely available alkenes, rather than from the less accessible and/or more expensive alkyl halide or organometallic C(sp3) synthons that are needed in a classical aryl/alkyl cross-coupling.









